TO THE EDITOR:
Peripheral blood flow cytometry is the test of choice to detect circulating neoplastic T-cells (Sézary cells) in patients with mycosis fungoides (MF) or Sézary syndrome (SS).1-4 These 2 clinically distinct subtypes of cutaneous T-cell lymphoma (CTCL) harbor neoplastic CD4+ T cells with largely identical immunophenotypic properties, and share a staging scheme that includes blood ratings based on absolute numbers of Sézary cells (B0: <250, B1: ≥250 and <1000, and B2: ≥1000 cells/μL).5 Flow cytometric identification of Sézary cells also plays a critical role in distinguishing SS from reactive erythroderma, and in the assessment of therapy response. Unfortunately, the laboratory identification of Sézary cells has remained challenging, given that their most frequent immunophenotypic characteristics (lack of CD7 and CD26 expression) are shared with common benign CD4+ T-cell subsets, whereas immunophenotypic properties specific for neoplasia (eg, diminished CD3 expression) are typically subtle or not identified.
We recently reported a novel flow cytometry strategy to rapidly confirm clonality on gated CD4+ T-cell subsets suspicious for Sézary cells6,7 using a single antibody against 1 of 2 mutually exclusive T-cell receptor constant β chains (TRBCs).8 We hereby present a semiautomated analysis to detect Sézary cells using an unsupervised clustering tool from the EuroFlow Consortium–sponsored analysis software (Infinicyt version 2.0; Cytognos, Salamanca, Spain)9 to automatically segment all CD4+ T cells into immunophenotypically distinct clusters and test each cluster for clonality based on TRBC1 expression. In a large cohort of clinical specimens, we show the superior test performance of this semiautomated approach compared to traditional gating strategies, and objectively report the complex immunophenotypic properties of Sézary cells as identified without a preconceived bias of an expected immunophenotype.
Peripheral blood from 136 patients with inflammatory dermatoses (143 samples), 191 patients with CTCL (343 samples), and 22 healthy donors (22 samples) was evaluated by flow cytometry at Mayo Clinic, Rochester, MN, using a single-tube Sézary cell panel (CD2, CD3, CD4, CD5, CD7, CD8, CD26, CD45, and TRBC1 [clone JOVI.1]) on a FACSCanto II flow cytometer (BD Biosciences), as previously described.6 Forty-five patients with inflammatory dermatoses had reactive erythroderma (33%); and 178 specimens from patients with CTCL were collected after systemic therapy or extracorporeal photopheresis (52%). Patients with MF were studied at stage I (125 samples), stage II (41 samples), stage III (30 samples), and stage IV (35 samples) of disease. Semiautomated analysis was performed in Infinicyt, including manual gating of CD4+/CD8– T cells per previously described strategies,3 automated density-based clustering of CD4+ T cells, automated detection of Sézary cell clusters exhibiting <15% or >85% TRBC1-positive cells, visual identification of Sézary cell clusters with dim TRBC1 expression, and expert gating upon suspicion of missed Sézary cells (Supplemental Figure 1, available on the Blood website). Absolute counts were calculated based on the absolute lymphocyte count obtained on a Sysmex XN-350 analyzer (Kobe). This study was approved by the Mayo Clinic Institutional Review Board.
Sézary cells were identified in 170 of 343 (50%) samples from patients with CTCL (range, 11-32 851 cells/μL). Small TRBC-restricted CD4+ T-cell subsets were also detected in a minority of benign samples (7 of 143 patients with inflammatory dermatoses; 4.9%; range, 11-155 cells/μL), a finding not associated with erythroderma (P = .28, χ2) and consistent with T-cell clones of uncertain significance6,10 (Figure 1A-D). Of 177 positive samples, 88% were automatically detected based on <15% or >85% TRBC1 positivity, 4% were visually identified based on dim TRBC1 expression, and 8% required expert gating of very small Sézary cell populations (range, 11-155 cells/μL). When compared to the gating strategy proposed by the European Organization for Research and Treatment of Cancer11 (EORTC; maximum of CD4+/CD7– and CD4+/CD26– T cells), our approach produced very similar Sézary counts in the B2 high tumor burden range but resulted in significant discrepancies in the B0-B1 range (Figure 1E). Notably, 18 of 62 samples (29%) rated B1 by EORTC gating lacked detectable clonal T cells by TRBC1 analysis, and 50 of 205 (24%) samples rated B0 by EORTC gating had small CD4+ T-cell clones by TRBC1 (Figure 1F). In samples from patients with inflammatory dermatoses, our semiautomated approach produced lower false Sézary cell counts (median, 0 vs 92 cells/μL; P < .0001, Mann Whitney test), more accurately classified samples as B0 (diagnostic specificity, 100% vs 92%; P = .0015, McNemar test), and confidently identified lower Sézary cell counts (limit of blank, 9 vs 345 cells/μL) compared to EORTC gating (Figure 1G). Moreover, EORTC Sézary cell counts on CTCL samples without detectable TRBC-restricted T cells closely mirrored those of patients with inflammatory dermatoses (Supplemental Figure 2A) consistent with poor Sézary cell discrimination by the EORTC method. Thus, our semiautomated approach to identify TRBC-restricted Sézary cells successfully overcame the limited specificity of the EORTC method and improved the confident identification of Sézary cells in the B0-B1 range.
Semiautomated detection of constant beta chain–restricted CD4+ T-cell clusters rapidly identifies Sézary cells, with improved specificity over traditional gating strategies. (A) Blood samples from healthy donors (left), patients with benign inflammatory dermatoses (middle), and patients with CTCL (right) were analyzed by flow cytometry using automated clustering and calculation of percentage TRBC1-positive events per cluster. Individual CD4+ T-cell clusters (dots) were classified as clonal (red) or nonclonal (blue) based on pre-established clonality thresholds (dotted lines) or monophasic TRBC1-dim expression on visual analysis. (B) Absolute numbers of Sézary cells (clonal CD4+ T cells) per microliter in patients with CTCL. (C) Results of blood ratings based on total clonal CD4+ T cells. (D) Number of clonal CD4+ T-cell clusters identified per sample on patients with CTLC. (E) Absolute numbers of Sézary cells estimated by clustering and TRBC1 expression compared to the gating strategy recommended by the EORTC. (F) Blood ratings estimated by the EORTC-recommended gating strategy compared to automated clustering and TRBC1 expression. (G) Diagnostic specificity for B1-B2 blood rating (%B0) and analytical specificity (limit of blank) of the EORTC-recommended gating strategy (left) compared to automated clustering and TRBC1 staining (right); based on patients with inflammatory dermatoses.
Semiautomated detection of constant beta chain–restricted CD4+ T-cell clusters rapidly identifies Sézary cells, with improved specificity over traditional gating strategies. (A) Blood samples from healthy donors (left), patients with benign inflammatory dermatoses (middle), and patients with CTCL (right) were analyzed by flow cytometry using automated clustering and calculation of percentage TRBC1-positive events per cluster. Individual CD4+ T-cell clusters (dots) were classified as clonal (red) or nonclonal (blue) based on pre-established clonality thresholds (dotted lines) or monophasic TRBC1-dim expression on visual analysis. (B) Absolute numbers of Sézary cells (clonal CD4+ T cells) per microliter in patients with CTCL. (C) Results of blood ratings based on total clonal CD4+ T cells. (D) Number of clonal CD4+ T-cell clusters identified per sample on patients with CTLC. (E) Absolute numbers of Sézary cells estimated by clustering and TRBC1 expression compared to the gating strategy recommended by the EORTC. (F) Blood ratings estimated by the EORTC-recommended gating strategy compared to automated clustering and TRBC1 expression. (G) Diagnostic specificity for B1-B2 blood rating (%B0) and analytical specificity (limit of blank) of the EORTC-recommended gating strategy (left) compared to automated clustering and TRBC1 staining (right); based on patients with inflammatory dermatoses.
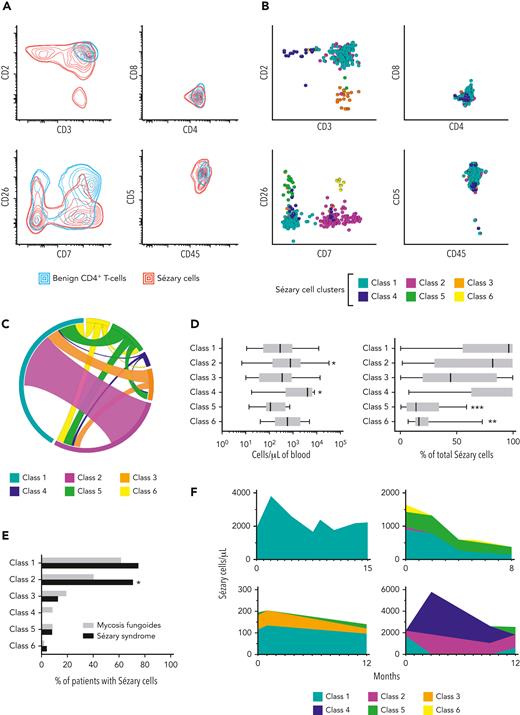
We then characterized the immunophenotypic properties of TRBC-restricted Sézary cells. Combined density maps of TRBC-restricted Sézary cells (Infinicyt) showed extensive overlap with benign CD4+ T cells from patients with inflammatory dermatoses on 2-dimensional projections of routinely evaluated antigens (Figure 2A), and on commonly used dimensionality reduction plots (Supplemental Figure 2B-C). Remarkably, there were no salient discriminatory features on the commonly used CD7 vs CD26 projection. The most distinct property of Sézary cells was variable loss of CD3 and CD2 expression, which was often but not always outside 2 standard deviations of the density contours of benign CD4+ T cells. Unsupervised k-means metaclustering (Qlucore Omics Explorer version 3.7, Qlucore) revealed up to 5 immunophenotypically distinct classes of Sézary cells containing at least 5 clusters each, with an additional class of interest (class 6) identified on supervised analysis: class 1 (CD7–/CD26–), class 2 (CD7+/CD26–), class 3 (CD2dim/–), class 4 (CD3dim/–), class 5 (CD7–/CD26+), and class 6 (CD7+/CD26+) (Figure 2B). Slightly dim CD3 expression was a feature commonly encountered in all classes, except for class 4 where CD3 was overtly decreased to absent. Although most (59%) samples had only 1 class of Sézary cells, the presence of multiple (2, 3, or 4) classes in a single specimen was a frequent finding (29%, 8%, and 4% of positive specimens, respectively) (Figure 2C). Classes 2 and 4, when present, tended to be more abundant than the prototypic class 1 Sézary cell (adjusted P < .05 for both); and classes 5 and 6 usually comprised a smaller fraction of total Sézary cells (adjusted P < .0001 and P < .01, respectively) (Kruskal-Wallis and Dunn tests) (Figure 2D). Interestingly, class 2 was more frequently encountered in SS compared to MF (P < .05), and class 4 was exclusively observed in MF (not statistically significant) (Fisher exact tests) (Figure 2E). Of 29 CTCL patients with more than 1 positive sample (average, 4.4 samples per patient; range, 2-11 samples), 15 (52%) showed a change in Sézary cell classes comprising the clonal subset, whereas shifts in the relative and absolute abundance of each Sézary cell class were frequently encountered (Figure 2F). Overall, these findings highlight the complex and frequently unstable immunophenotypic properties of Sézary cells which often overlap with those of reactive CD4+ T cells, making them challenging to identify by comprehensive immunophenotyping alone without clonality assessment.
Immunophenotypic features of constant beta chain–restricted Sézary cells showing complex multicluster composition and overlap with benign T-cell subsets. (A) Density plots showing extensive immunophenotypic overlap between constant beta chain–restricted Sézary cells from patients with CTCL (red lines), and benign CD4+ T cells from patients with inflammatory dermatoses (blue lines). Double lines depict 2 standard deviations. (B) Metaclustering (K-means) of constant beta chain–restricted Sézary cell clusters showing distinct immunophenotypic subtypes on 2-dimensional projections (described as class 1-6). (C) Circos plot depicting the incidence (segments) and observed combinations (ribbons) of Sézary cell metaclusters. (D) Contribution of each immunophenotypic class of Sézary cell to the total Sézary cell count based on absolute numbers of cells per microliter (left) and percentage of total Sézary cells (right). Box and whiskers: median, interquartile range and total range. Asterisks (∗), (∗∗), and (∗∗∗) indicate P < .05, P < .01, and P < .0001, respectively, compared to class 1. (E) Incidence of each immunophenotypic class of Sézary cell (first positive sample only) in patients with mycosis fungoides (grey bars) and Sézary syndrome (black bars). Asterisk (∗) indicates P < .05. (F) Examples of patients with Sézary syndrome (top) and mycosis fungoides (bottom), showing variability of Sézary cell metacluster composition and abundance on serial samples.
Immunophenotypic features of constant beta chain–restricted Sézary cells showing complex multicluster composition and overlap with benign T-cell subsets. (A) Density plots showing extensive immunophenotypic overlap between constant beta chain–restricted Sézary cells from patients with CTCL (red lines), and benign CD4+ T cells from patients with inflammatory dermatoses (blue lines). Double lines depict 2 standard deviations. (B) Metaclustering (K-means) of constant beta chain–restricted Sézary cell clusters showing distinct immunophenotypic subtypes on 2-dimensional projections (described as class 1-6). (C) Circos plot depicting the incidence (segments) and observed combinations (ribbons) of Sézary cell metaclusters. (D) Contribution of each immunophenotypic class of Sézary cell to the total Sézary cell count based on absolute numbers of cells per microliter (left) and percentage of total Sézary cells (right). Box and whiskers: median, interquartile range and total range. Asterisks (∗), (∗∗), and (∗∗∗) indicate P < .05, P < .01, and P < .0001, respectively, compared to class 1. (E) Incidence of each immunophenotypic class of Sézary cell (first positive sample only) in patients with mycosis fungoides (grey bars) and Sézary syndrome (black bars). Asterisk (∗) indicates P < .05. (F) Examples of patients with Sézary syndrome (top) and mycosis fungoides (bottom), showing variability of Sézary cell metacluster composition and abundance on serial samples.
In conclusion, we show the optimal test performance of a semiautomated flow cytometry strategy for objectively identifying Sézary cells based on the detection of immunophenotypically distinct CD4+ T-cell clusters exhibiting a clonal TRBC1 expression pattern. This approach results in improved test specificity and confident Sézary cell count reporting in the B0-B1 range over the proposed EORTC strategy. In addition, we describe the immunophenotypic spectrum of Sézary cells based on the unbiased identification of clonal CD4+ T cells in patients with CTCL, including demonstration of the extensive immunophenotypic overlap with reactive T cells and a complex mixture of immunophenotypically distinct neoplastic subsets. Limitations of this strategy include inability to detect very rare circulating CD8+ and CD4+/CD8+ CTCLs (excluded per gating design), and subjectivity in discerning small CD4+ T-cell clones of uncertain significance from low-level/minimal involvement by CTCL. Overall, our results support the upfront and standardized assessment of TRBC1 expression in the routine flow cytometric identification of Sézary cells.
Authorship
Contribution: P.H., H.O., and G.O. designed the study; P.H. and G.O. performed case search and flow cytometric analysis; P.H. analyzed the data and wrote the manuscript; J.S., G.O., and H.O. contributed to the data analysis design; and D.J., M.S., and J.S. edited the manuscript and provided project support and expert knowledge.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pedro Horna, Mayo Clinic, 200 First Street SW, Rochester, MN 55905; e-mail: horna.pedro@mayo.edu.
References
Author notes
The online version of this article contains a data supplement.