Abstract
Introduction: While most patients with newly diagnosed multiple myeloma (NDMM) respond to first line treatment, most patients with multiple myeloma will eventually relapse. For treatment of patients with relapsed multiple myeloma (RMM), regimens may contain combinations that include a CD38 monoclonal antibody, proteosome inhibitor, an immunomodulatory (IMID) agent, and corticosteroids. As most induction therapies for NDMM include bortezomib, lenalidomide, and dexamethasone (VRD), therapies for second- and third-line treatment often include either a carfilzomib- or daratumumab-containing regimen. However, the optimal treatment sequence at first relapse remains to be addressed. Herein, we present a multi-center retrospective study evaluating if a carfilzomib-first (CF) versus daratumumab-first (DF) containing regimen in the second line setting for RMM affects second progression free survival (PFS2) or OS.
Methods: We performed a multicenter retrospective study of 5 academic centers in the United States. Chart review of patients who had RMM and who received either carfilzomib or daratumumab based regimen as the backbone of their second line (2L) regimen followed by the opposite drug in the subsequent regimen between January 2010 to December 2018. Pts must have received either carfilzomib or daratumumab as their second line regimen. If a third line (3L) regimen is required (i.e., if CF treatment is 2L, then daratumumab must have been given as 3L. Patients who received 2L therapy with both drugs or had prior exposure to either drug were excluded. The primary endpoint is PFS2, defined as time from 2nd line treatment initiation to next disease progression or death. OS is the secondary endpoint of the study. Descriptive statistics, including medians and inter-quartile ranges for continuous parameters, as well as percentages and frequencies for categorical parameters, were presented. Cox regression was applied to assess the association between treatment and time to event outcome with the adjustment of potential confounders, though some additional variables may not be accounted for.
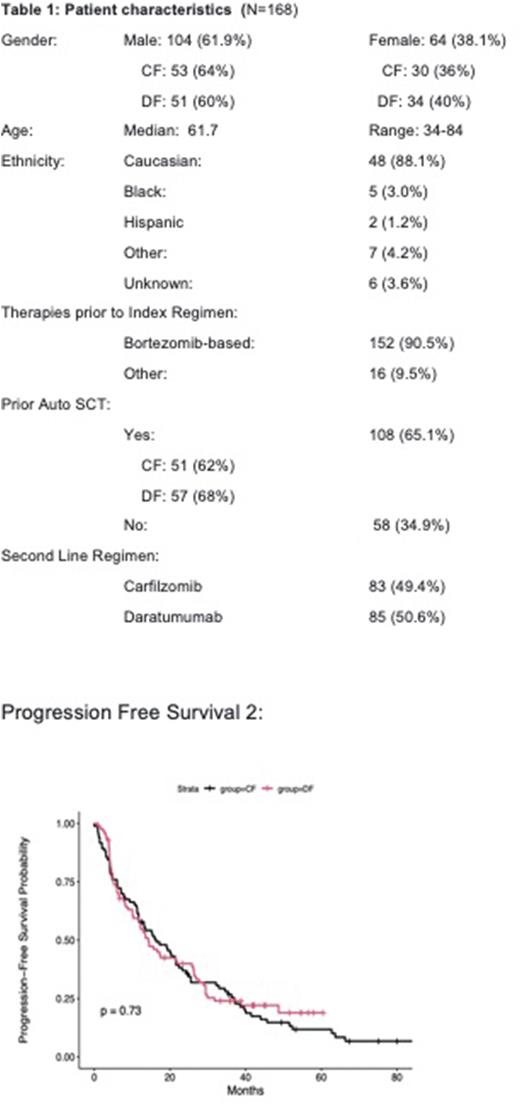
Results: At total of 168 patients were included in this study. Baseline patient demographics were summarized in Table 1 . Most patients were male (Total: 104 (61.4%); CF: 64% and DF: 60%)), and most were of Caucasian descent (88.1%). The median age of diagnosis was 62 (IQR: 34-84). Median time to follow-up was 42 mos. in the CF group and 37 mos in the DF group. . Patients who required treatment with 3L regimen, 53.5 (N=74) would go on to have progressive disease. In univariate analysis, there was no detectable difference in PFS2 (p=0.73) (Figure 1) or OS (p=0.57) between the two groups.
Conclusion: In this retrospective study of patients with RMM, there was no difference in PFS2 or OS in whether carfilzomib or daratumumab based regimens were selected in the second line treatment setting. Therefore, either regimen remains reasonable in the second line setting, and there is no optimal sequence in the current era of available therapies in RMM. Additional data is needed to determine if combining the two drugs at first relapse results in improved outcomes.
Disclosures
Sengsayadeth:Amgen: Research Funding. Schmidt:Janssen: Consultancy; Sanofi: Consultancy. Silbermann:Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Chhabra:Amgen: Research Funding; GlaxoSmithKline: Honoraria; Janssen: Research Funding; Sanofi: Research Funding. Kumar:AbbVie,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive,: Membership on an entity's Board of Directors or advisory committees, Research Funding; KITE,: Research Funding; MedImmune/Astra Zeneca,: Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck,: Research Funding; Novartis,: Research Funding; Roche: Research Funding; Sanofi: Research Funding; Oncopeptides: Other: Independent review committee. Costa:AbbVie: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; Genentech: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.