Abstract
Background: Graft-versus-host disease (GvHD), mediated by the activation of donor T cells against recipient major histocompatibility complex antigens, is a major source of morbidity and mortality following allogeneic hematopoietic stem cell transplant (allo-HSCT). Most treatments for GvHD focus on limiting donor T cell expansion or altering the phenotype of the donor T cells. PI3K/Akt/mTOR signaling pathway is crucial in T cell activation and effector function and blocking PI3K/mTOR signaling can prevent GvHD (Herrero-Sánchez, 2016). However, few studies have explored the immunosuppressive effects of PI3K inhibitors. Duvelisib, a dual inhibitor of PI3K delta and PI3K gamma isoforms, has been approved by FDA to treat CLL/SLL. Here, we studied the efficacy of duvelisib in limiting GvHD using murine allo-BMT models. Our study shows that duvelisib reduced GvHD and improved the survival of allo-transplant recipients by inhibiting T cell expansion in vivo.
Methods: We assessed T cell expansion by utilizing Bioluminescent imaging (BLI) and monitored the survival of recipient mice. To modulate GvHD in our murine allo-BMT model, C57BL/6 recipient mice received a 9 Gy dose of myeloablative irradiation on day -1 and were transplanted on day 0 with 5 x 106 T cell-depleted (TCD) bone marrow cells from FVB donor mice and 0.5 x 106 T cells from luciferase positive FVB mice. We treated recipient mice with duvelisib (25mg/kg) or incipient diluent by daily gavage for 6 weeks starting from post-transplant day 1. We weighed the mice twice a week and performed IVIS imaging weekly. To explore T cell expansion by flow cytometry, we established another GvHD model in which C57BL/6 recipient mice received a 10 Gy dose of myeloablative irradiation on day -1 and were transplanted on day 0 with 5 x 106 TCD bone marrow cells and 5 x 106 T cells from Balb/c mice. We treated the mice until day 22, euthanized them, and harvested the splenocytes for flow cytometry analysis, measuring T cells subset and intracellular transcription factors and cytokines by flow cytometry.
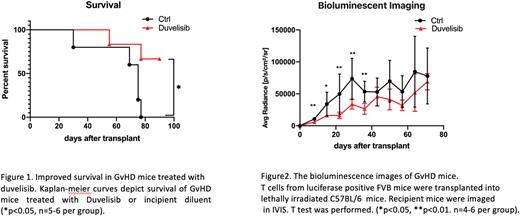
Results: Recipient mice in the duvelisib treatment group had better survival (66.7%) than mice in the control group (0%) (Fig 1). BLI data showed less T cell expansion in the duvelisib treatment group than in the control group (p<0.01 on day 8, 22, 29, and 37; p<0.05 on day 15; n=5 in the control group before day30 and n=4 after day30; n=5-6 per treatment group) (Fig 2). Weight gain after transplant of mice in the duvelisib treatment group was increased compared with mice in the control group (p<0.01 at day 8, n=5-6 per group). Immunophenotyping splenocytes on day 22 post-transplant with flow cytometry showed a decreased percentage of T cells in the spleen with duvelisib treatment on day 22, consistent with results of BLI imaging data in a second allo-BMT model (p<0.05, n=5). CD4+ T cells from mice in the duvelisib treatment group tended to have higher expression of GATA3 and IL-10 and lower expression of RORγt, TNF, and IL17 compared with the control group (p=NS, n=4-5 per group). Furthermore, after day 60, donor T cell expansion increased in duvelisib-treated mice, and these mice continued to gain weight without clinical signs of GvHD, suggesting that in vivo exposure to duvelisib leads to tolerance or deletion of alloreactive T cells.
Conclusion: Our data suggest that duvelisib treatment can inhibit T cell expansion in vivo and reduce GVHD-mediated mortality. Duvelisib treatment appears to favor Th2 cell polarization in donor T cells, but additional experiments are needed to confirm statistical significance. Inhibition of PI3K delta/gamma isoforms is a potential strategy for the treatment of T cell-mediated auto-immune and inflammatory disorders. Ongoing experiments evaluating the cytokine profile of T cells and correlating gene expression analysis with histology of GVHD organs will elucidate mechanisms of duvelisib-mediated GVHD prevention.
Disclosures
Waller:Verastem Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orca Bio: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.