Abstract
Background and aims:
Chronic graft vs host disease (cGVHD) is a common complication following allogeneic haematopoietic stem cell transplantation (HSCT), occurring in 30-70% of recipients receiving T cell replete grafts [1]. While corticosteroids are the backbone of first line therapy for cGVHD, less than 20% of patients achieve a durable partial or complete response with this therapy alone [2]. Despite the advent of novel agents for second line therapy of steroid refractory cGVHD (SR-cGVHD), disease progression is not uncommon highlighting an unmet clinical need.
GVHD is believed to be mediated by donor derived alloreactive B and T cells. Ibrutinib is a first in class Bruton tyrosine kinase (BTK) inhibitor, with additional inhibitory effects on other tyrosine kinases such as interleukin-2 inducible kinase (ITK). BTK and ITK play important roles in B and T cell stimulatory signalling pathways respectively. Inhibition of these pathways in alloreactive B and T cell clones may ameliorate cGVHD, and in recent phase 1b/II clinical trials in SR-cGVHD ibrutinib has demonstrated response rates of 60-70% [3].
The outcomes in real world clinical practice may differ from those observed in trials, as patients may have received more lines of cGVHD therapy or have different comorbidities affecting tolerability. We assessed the efficacy and tolerability of ibrutinib given for SR-cGVHD in a real world cohort of Australian patients.
Methods: A retrospective review of pharmacy dispensing records was undertaken at six allogeneic bone marrow transplantation centres across Australia between Jan 2018 and Jan 2020 to identify patients treated with ibrutinib on compassionate access for SR-cGVHD. Clinical records of identified patients were reviewed to extract data using standardised case report forms including transplant details, cGVHD manifestations and treatment history, cGVHD response to ibrutinib (as per NIH cGVHD consensus criteria [4]), adverse events, relapse and survival.
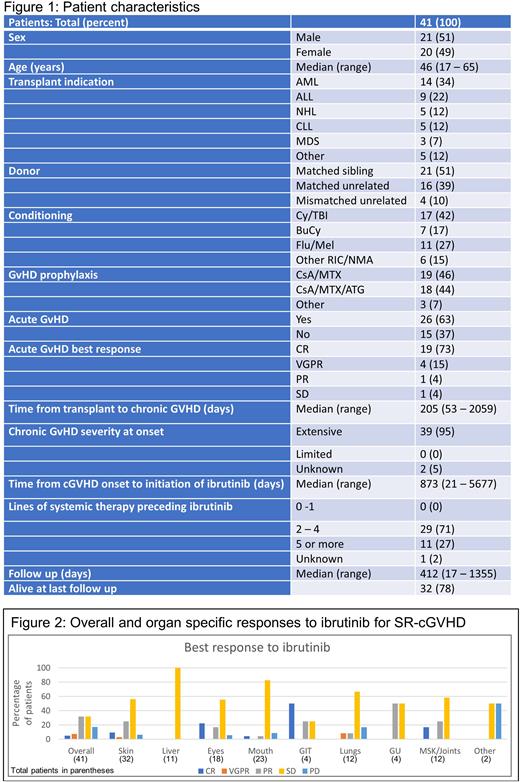
Results: Demographic details of the 41 eligible patients are outlined in Figure 1. The majority (95%) had extensive cGVHD with a median of 3 lines of systemic therapy (range 2-8) preceding ibrutinib. The median starting dose of ibrutinib was 420mg daily (range 140 - 420mg daily), with an overall response rate of 44%, with a complete response seen in 5% (Figure 2). Corticosteroid dosing information was available for 20 patients, with a mean reduction in dose of 44% during ibrutinib treatment. There were a total of 50 adverse events in 22 patients, of which 48% were infections. After a median follow up of 412 days, 46% of patients were continuing ibrutinib, with the most common reasons for cessation being toxicity (32%) or progressive cGVHD (27%). At last follow up, 32/41 patients were alive, with causes of death being cGVHD (6 patients), infection (2 patients) and secondary malignancy (1 patient).
Conclusion: Ibrutinib demonstrates modest activity in a real world setting of heavily pre-treated patients with SR-cGVHD. Toxicities, particularly those related to infections, appear to limit long term use. This study highlights the potential limitations of the use of ibrutinib for SR-cGVHD in a real world context, particularly with reference to tolerability in heavily pre-treated patients.
References 1. Lee, S.J., Have we made progress in the management of chronic graft-vs-host disease? Best Pract Res Clin Haematol, 2010. 23(4): p. 529-35.
2. Martin, P.J., et al., An endpoint associated with clinical benefit after initial treatment of chronic graft-versus-host disease. Blood, 2017. 130(3): p. 360-367.
3. Waller, E.K., et al., Ibrutinib for Chronic Graft-versus-Host Disease After Failure of Prior Therapy: 1-Year Update of a Phase 1b/2 Study. Biol Blood Marrow Transplant, 2019. 25(10): p. 2002-2007.
4. Lee, S.J., et al., Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant, 2015. 21(6): p. 984-99
Disclosures
Hamad:Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Grigg:Novartis: Membership on an entity's Board of Directors or advisory committees. Gottlieb:Haemalogix Ltd: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.