Abstract
Background Primary refractory acute myeloid leukemia (prAML) is a poor prognosis disease for which allogeneic hematopoietic stem cell transplantation (allo-HSCT) is the only curative option currently available. We aimed to estimate outcomes in the recent period, to analyze how different donor types may have impacted survival outcomes and to identify the most relevant predictive factors for allo-HSCT in prAML.
Methods We conducted a retrospective multicenter study of adult patients reported to the European Society for Blood and Marrow Transplantation (EBMT) registry who received an allo-HSCT between 2015 and 2020 for prAML. We considered HSCT performed from matched sibling donors (MSD), 10/10 unrelated donors (UD 10/10), 9/10 unrelated donors (UD 9/10) and haploidentical donors. Primary endpoint was leukemia-free survival (LFS), secondary endpoints were overall survival (OS), relapse incidence (RI) and non-relapse mortality (NRM). A Cox proportional hazards model was used for multivariate analysis.
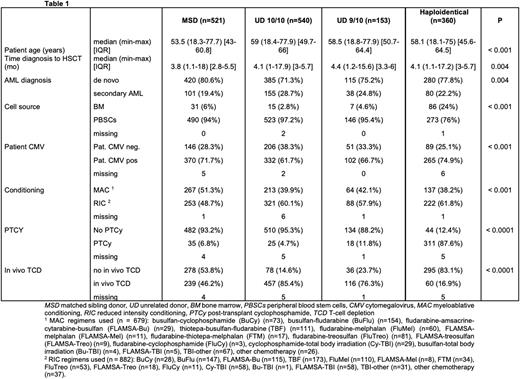
Results We included 1574 patients. Median age was 56.9 years (range 18.1-77.9). Cytogenetic risk was intermediate in 855 (54.3%), adverse in 679 (43.2%) and favorable in 40 (2.5%) patients. Donor type was distributed as follows: MSD n = 521 (33.1%), UD 10/10 n = 540 (34.3%), UD 9/10 n = 153 (9.7%), haploidentical n = 360 (22.9%). Table 1 summarizes patient and transplant characteristics. Patients allografted from an MSD were younger, allografted faster after diagnosis, more often received myeloablative conditioning (MAC) (details in Table 1) and had less secondary AML. Use of post-transplant cyclophosphamide, and bone marrow as a stem cell source were more frequent in haploidentical transplants.
LFS at 2 years was significantly lower in haploidentical transplants (27% [95%CI: 22-32.2]) compared to MSD (35.2% [95%CI: 30.6-39.9]), to UD 10/10 (37.6% [95%CI: 33.2-42.1]) and to UD 9/10 (35.2% [95%CI: 27.1-43.5]) (p=0.003). Haploidentical transplants also showed inferior OS at 2 years: 31.2% (95%CI: 25.8-36.8) compared to 40.9% (95%CI: 35.9-45.8) in MSD, to 45.7% (95%CI: 41.1-50.2) in UD 10/10 and to 43% (95%CI: 34.5-51.3) in UD 9/10 (p=0.001). In the UD 9/10 group we observed the lowest RI (33.5% [95%CI: 25.6-41.6], 46.7% [95%CI: 41.8-51.4] in MSD, 42.7% [95%CI: 38.2-47.1] in UD 10/10, 46.8% [95%CI: 41.1-52.3] in haploidentical) (p=0.013), and the highest NRM (31.2% [95%CI: 23.6-39.2], 18.1% [95%CI: 14.6-21.9] in MSD, 19.7% [95%CI: 16.3-23.3] in UD 10/10, 26.2% [95%CI: 21.6-31.1] in haploidentical) (p=0.001). Of note, LFS at 2 years was reduced for adverse cytogenetic risk (26.1% for adverse, 39.8% for intermediate and 51.9% for favorable risk) (p=0.001) and significantly differed between reduced intensity conditioning (RIC) and MAC (37.4% and 29.8%, respectively) (p=0.033).
In multivariate analysis, factors predicting poor LFS were haploidentical donor (hazard ratio [HR] 1.22 [95%CI: 1.01-1.48], p=0.043), adverse cytogenetic risk (HR 1.73 [95%CI: 1.51-1.99], p<0.0001) and time from diagnosis to HSCT (HR 1.03 [95%CI: 1.01-1.06], p=0.009), whereas factors associated with a better LFS were RIC conditioning (HR 0.85 [95%CI: 0.73-0.98], p=0.025) and good Karnofsky performance status (KPS) ≥ 80 (HR 0.74 [95%CI: 0.62-0.88], p=0.0009). Haploidentical transplant correlated with lower OS (HR 1.3 [95%CI: 1.06-1.61], p=0.014) and higher NRM (HR 1.69 [95%CI: 1.18-2.41], p=0.004) and adverse cytogenetic risk was associated with higher RI (HR 1.9 [95%CI: 1.6-2.24], p<0.0001), higher NRM (HR 1.47 [95%CI: 1.15-1.87], p=0.002) and lower OS (HR 1.82 [95%CI: 1.57-2.11], p<0.0001). Moreover, increased age predicted higher NRM (HR 1.31 [95%CI: 1.17-1.47], p<0.0001) and lower OS (HR 1.09 [95%CI: 1.02-1.16], p=0.006).
Conclusions Our study identified risk factors for survival outcomes in the challenging setting of allo-HSCT for prAML. The most relevant factors are adverse cytogenetic risk and haploidentical donor, both associated with worse outcomes. Of note, the group of haploidentical transplants presented the highest rate of bone marrow used as a stem cell source, with a possible impact on survival outcomes. Longer time from diagnosis to HSCT negatively impacted LFS, whereas use of RIC and KPS ≥ 80 showed a higher LFS. As concerns secondary endpoints, unrelated donors achieved better OS compared to other donors, and UD 9/10 showed the lowest RI at the expense of the highest NRM.
Disclosures
Labopin:Jazz Pharmaceuticals: Honoraria. Kröger:Takeda: Consultancy, Honoraria; Sanofi: Honoraria; Kite: Honoraria; Neovii: Honoraria, Research Funding; Riemser: Research Funding; DKMS: Research Funding; Amgen: Honoraria; BMS: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Jazz: Honoraria. Finke:Riemser Pharma: Research Funding. Stelljes:MSD: Consultancy, Honoraria; Amgen: Consultancy; Medac: Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Jazz: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Kite: Consultancy, Honoraria. Ganser:Novartuis: Consultancy; Jazz Pharmaceuticals: Consultancy; Celgene: Consultancy. Einsele:BMS/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Grants, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grants; Sanofi: Consultancy, Honoraria, Research Funding; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Other: travel grants. Schetelig:Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Forcade:Sanofi: Other: Travel Support; GSK: Speakers Bureau; Novartis: Speakers Bureau; Jazz: Other: Travel Support, Speakers Bureau; Gilead: Other: Travel Support, Speakers Bureau; MSD: Other: Travel Support. Ciceri:Kite Pharma: Consultancy. Mohty:Adaptive Biotechnologies: Honoraria; Bristol Myers Squibb: Honoraria; Celgene: Honoraria; Novartis: Honoraria; Jazz Pharmaceuticals: Honoraria, Research Funding; Astellas: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Oncopeptides: Honoraria; Pfizer,: Honoraria; GSK: Honoraria; Sanofi: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Gilead: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.