Abstract
Background:TP53 gene mutations are detected in approximately 10%-20% of patients with de novo myelodysplastic syndromes (MDS) or acute myeloid leukemia (AML) and 30%-40% of patients with therapy-related disease. TP53 mutated MDS/AML are associated with a dismal prognosis when treated with intensive chemotherapy alone. Even after allogeneic stem cell transplant (SCT), TP53 mutated MDS and AML patients are shown to have low survival rates.
In this single center retrospective analysis, we aimed to investigate the prognostic value of TP53 mutations in patients with MDS/AML that undergo SCT and to identify other variables such as conditioning regimen, use of post-transplant maintenance, donor selection or hematopoietic cell transplantation-specific comorbidity index (HCT-CI) had any impact on the post-transplant outcomes.
Methods: Patients with a diagnosis of MDS or AML who received first SCT between January 2013 through April 2022 with available pre-transplant genetic profile obtained from next generation sequencing of genes, and confirmed to have TP53 mutation, were included in the retrospective analysis. Disease risk factors were categorized by the revised International Prognostic Scoring System (R-IPSS) for MDS and European Leukemia Net (ELN)for MDS and AML respectively. When using ELN classification, cytogenetic and molecular data was used as instructed other than TP53 to able to identify different risk groups for SCT outcomes. Primary outcome of interest was risk of relapse-free survival (RFS). MDS and AML patients were analyzed separately.
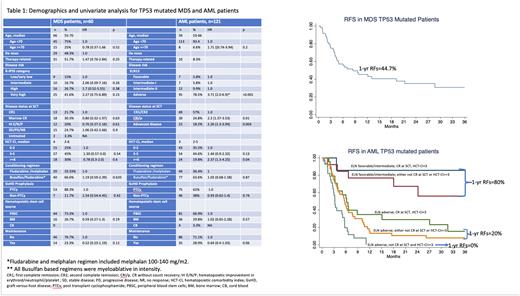
Results: Within the study period, 181 patients (60 MDS and 121 AML) were eligible to be included in this analysis. As shown in the table 1, the study cohort with TP53 mutation was a high risk one; disease risk groups were very-high and high by IPSS-R in 78.4% of MDS, and adverse risk by ELN in 78.5%% of AML patients, respectively. Median age was 66 and 59 in MDS and AML patients and HCT-CI was >2 at SCT in 75% of MDS and 64.5% of AML patients respectively.
In MDS patients, median RFS was 8.4 months with 1-year estimate of 44.7% (Figure 1a). By univariate analyses, none of the disease and transplant related characteristics were found to have a prognostic impact on RFS.
In AML patients, median RFS was 5.7 months with 1-year estimate of 27.4%. By univariate analyses, as shown in Table 1, adverse risk category by ELN, complete remission without count recovery (CRi/p), advanced disease at transplant and HCT-CI >2 were significant predictors of decreased RFS. The use of post-transplant maintenance was shown to improve RFS.
Multivariate regression (MV) in AML patients revealed that adverse risk by ELN (HR=3.6, CI=1.9-6.8, p<0.001), disease status at SCT other than CR1/CR2 (HR=2.1, CI=1.4-3.3, p<0.001) and HCT-CI >2 (HR=1.9, CI=1.9-6.9, p<0.001) were poor risk factors for worse RFS. Maintenance treatment lost its significance to improve RFS with MV (HR=0.76; CI=0.47-1.24, p=0.3). Based on MV, 3 mutually exclusive risk groups for RFS were identified (Figure 1b). The best group included patients with favorable/intermediate risk by ELN, with no more than one other risk factor (CRi/p or advanced disease at SCT or HCT-CI >2) with 1-year RFS of 80%. On the other hand, patients with adverse risk by ELN, transplanted in CRi/p or with advanced disease and had HCT-CI>2 represented a very poor group with 1-year RFS of 0%. Adverse risk ELN patients had slightly better RFS if they had only one other high-risk feature with 1-year estimate of 20%.
Overall survival at 1-year in MDS and AML patients were 57.3% and 36.4% respectively. The cause of death was relapse of disease in 65% of MDS and 63% in AML patients. Median time to relapse was 135 days, and incidence of disease relapse was very high as 31.2% at 6 months and 49.5% at 1-year in MDS and 30% at 6 months and 47.9% at 1-year in AML patients.
Conclusion: TP53 mutated MDS and AML patients continue to represent one of the worst prognostic groups for outcome expectations after transplant. In our analysis, the conditioning regimen, stem cell source and use of post-transplant maintenance therapies did not lead to improved outcomes. On the other hand, among AML patient, we were able to identify a subgroup with favorable outcomes indicating that other disease related characteristics need to be taken into account while planning transplant in this population. Innovative strategies to improve outcomes are urgently needed for TP53 mutated MDS and AML patient.
Disclosures
Popat:Incyte: Research Funding; Novartis: Research Funding; Bayer: Research Funding; Abbvie: Research Funding. Kebriaei:Pfizer: Consultancy; Amgen: Research Funding; Jazz: Consultancy; Kite: Consultancy; Ziopharm: Research Funding. Alousi:Prolacta: Consultancy; Genetech: Consultancy; Incyte: Honoraria, Research Funding; Sanofi / Kadmon: Honoraria; Mallinkrodt: Honoraria. Mehta:Syndax: Research Funding; Orca Bio: Research Funding. Shpall:NY blood center: Consultancy; Navan: Consultancy; axio: Consultancy; Fibroblasts and FibroBiologics: Consultancy; Takeda: Patents & Royalties; Bayer: Honoraria; adaptimmune: Consultancy; Affimed: Other: License agreement. Champlin:General Oncology: Other: Data Safety Monitoring Board; Bluebird: Other: Data Safety Monitoring Board; Actinium: Consultancy; Kadmon: Consultancy; Cell Source Inc.: Research Funding; Omeros: Consultancy; Johnson &Johnson: Consultancy. Oran:ASTEX: Research Funding; AROG: Research Funding.
OffLabel Disclosure:
In this retrospective analyses, some patients recived post transplant maintence treatment with various drugs including hypomethylating agents and venetoclax.
Author notes
Asterisk with author names denotes non-ASH members.