Abstract
Background: Chimeric Antigen Receptor (CAR) T-cell therapy is available as a salvage option for patients with relapsed/refractory B-cell lymphomas (DLBCL, FL, and MCL). Historically, most CAR T-cell therapy has been administered in the inpatient setting due to the potential need to closely monitor or treat cytokine release syndrome (CRS) and neurologic toxicity (NT). However, the introduction of rigorous outpatient monitoring programs incorporating nurse-driven education, patient/caregiver-driven after-hours reporting, and prophylactic corticosteroids may enable outpatient administration and monitoring of CAR T-cell in a large proportion of patients. Outpatient monitoring can improve quality of life for the patient as well as reduce healthcare costs.
Objective: Describe clinical outcomes in a cohort of patients who received a variety of commercial CAR T-cell therapies and were subsequently monitored in the outpatient setting.
Study Design: This is a retrospective analysis of 20 patients treated with outpatient CAR T-cell therapy between February 2021 and March 2022 with all patients having at least 100 days of post-infusion follow up. Additional data points included progression free survival, overall survival, HCT-CI score, prior lines of treatment, ECOG scores (before and after treatment), and incidence of cytokine release syndrome and neurological toxicity. Responses to treatment were determined based on radiographic tumor evaluation by metabolic response with PET-CT. Patients were monitored with daily RN triage visits and in the absence of complications, were seen twice weekly by an advanced practice provider (APP) or MD. Patients did not use remote monitoring devices and both patients and caregivers were encouraged to self-report any symptoms of CRS or NT to a 24 hour on-call APP/MD.
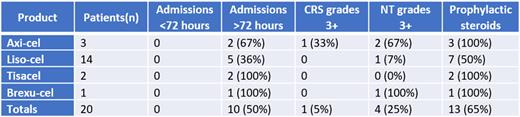
Results: Of the 20 patients in the cohort, 2, 3, 14, and 1 patients received tisagenlecleucel, axicabtagene ciloleucel, lisocabtagene maraleucel, and brexucabtagene autoleucel, respectively. The median age was 69.5 (range 46-81). 60% of patients were male. 65% (13/20) of patients were treated with prophylactic dexamethasone. 55% of patients had an IPI score of 3-4. 25% (5/20) of patents had an ECOG score of 2 or greater. Median HCT-CI score was 1 and ≥2 in 9/20 (45%) patients (range 0-5). Overall, 10/20 (50%) patients were admitted in the first month of therapy (all initially for management of CRS), and no patients were admitted within the first 72 hours after infusion. The incidence of CRS was 11/20 (55%) with grade 3+ CRS of 5%. The incidence of NT was 9/20 (45%), with grade 3+ NT of 20%. Progression free survival in the cohort was 65% at 6 months and 60% at 1 year, and overall survival was 85% and 75%, respectively. 2 (10%) patients died prior to day 30 of treatment, one due to MRSA bacteremia (which developed >1 week into hospital admission), one due to progressive disease. No patients were admitted directly to the ICU.
Conclusions: In a single-center cohort of patients who received CAR T-cell therapy and were subsequently monitored in the outpatient setting without remote monitoring devices, only half of the patients were admitted in the 1st month of therapy and no patients were admitted within the first 72 hours after infusion. 2 patients died in the first month of treatment for reasons unlikely to be related to outpatient monitoring.
Disclosures
Hunter:ADC Therapeutics: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Notable Labs: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Kite Pharma: Consultancy, Honoraria, Speakers Bureau.
OffLabel Disclosure:
Usage of prophylactic steroids with CAR T-cell therapy (only labeled use is with axicabtagene ciloleucel)
Author notes
Asterisk with author names denotes non-ASH members.