Abstract
Introduction: The multifactorial and complex pathophysiology of pulmonary embolism (PE) involves dysregulation of hemostatic system including fibrinolytic imbalance, endothelial compromise, and generation of thrombotic mediators, along with cellular defects and hemodynamic derangement. These pathophysiologic processes results in the generation of thrombo-inflammatory biomarkers reflecting the endogenous hemostatic imbalance. Cancer is prevalent in PE patients and is considered as an added risk factor for the adverse outcomes. Thrombo-inflammatory biomarker profiling provide means to risk stratify PE patients. This study aimed to profile several of these biomarkers in normal individuals and PE patients and further demonstrate their additional amplification in patients with cancer as a co-morbidity.
Materials & Method: Four hundred male and female patients of 18 years or older age, were included through enrolment in conjunction with an IRB approved project by the pulmonary embolism response team (PERT) registry. Diagnosis of PE was confirmed by computed tomography (CT), angiography or ventilation perfusion imaging. Blood samples were drawn with in 24-72 hours of confirmed diagnosis of acute PE. Normal human plasma (NHP) samples were obtained from 50 healthy individuals including 25 male and 25 female and used for referencing purposes. All of the plasma samples were analyzed for D-Dimer, plasminogen activator inhibitor-1 (PAI-1) antigen, tissue-type plasminogen activator (tPA), activated thrombin-activatable fibrinolysis inhibitor (TAFI-a), von Willebrand factor (vWF), c-Reactive Protein (CRP) and interleukin-6 (IL-6) were measured by commercially available ELISA methods. Microparticles were measured by using a chromogenic functional method. Individual biomarkers were compared to normal plasma and further stratified on cancer status and PE severity. Results are presented as mean±SD and fold increase. For the degree of association, GraphPad Prism Software was used.
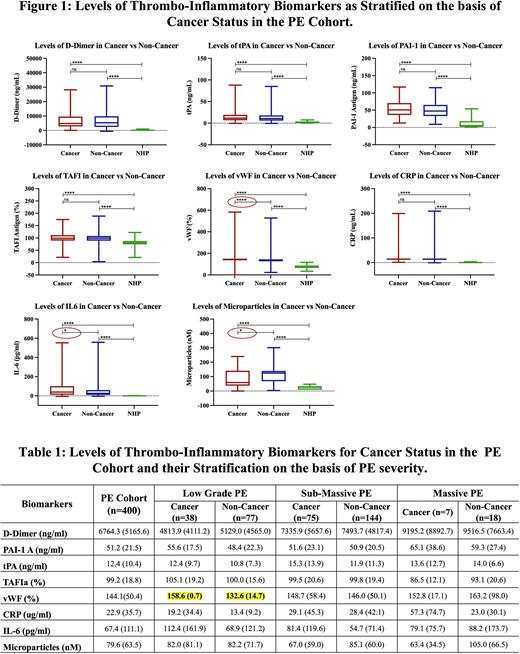
Results: PE patients exhibited varying levels of upregulation of all of the individual biomarkers compared to normal's, D-Dimer (37.05-fold), PAI-1 antigen (3.95-fold), tPA (4.69-fold), TAFI (1.21-fold), vWF (1.89-fold), CRP (35.34-fold), IL-6 (21.62-fold), and microparticles (3.04-fold) increase in PE patients with reference to NHP. When the PE group stratified into cancer and non-cancer patients, most of the biomarkers except D-Dimer and microparticles showed varying degree of modest elevation in the cancer group (figure 1). vWF, IL6 and microparticles levels were found to be further amplified in PE group with cancer. Of these three biomarkers, only vWF remained amplified when stratified by PE severity in patients with cancer, however this change was only observed in the low-grade PE patients (table 1). The IL-6 levels showed increased trend in the low-grade and sub-massive patient group, however because of the wide scatter, it was not significant.
Conclusion: These studies demonstrate that thrombo-inflammatory biomarkers are upregulated in PE patients. Furthermore, PE patients with cancer exhibit amplified responses for vWF, IL-6 and microparticles levels suggesting their role in mediating the pathogenesis involving endothelial, inflammatory and thrombogenic mechanisms. Among the biomarkers studied, vWF is central to thrombo-inflammatory process and is distinct in PE cohort with cancer, despite taking into account the lower PE severity. These studies suggest that selective biomarker profiling of thrombo-inflammatory biomarkers may be valuable in the risk stratification and therapeutic management of PE patients at large.
Disclosures
Monreal:Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: Presented to congress, Research Funding; Rovi: Research Funding; Leo Pharm: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.