Abstract
Background: Primary Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder due to decreased platelet count (PLT). Patients (Pts) with ITP face manifold challenges owing to the multifarious burden of living with the disease and its treatment-related complications. Corticosteroids used as first-line therapy in both adults and children do not improve quality of life or patient-related outcomes. The real-world experience data on the treatment outcomes of ITP pts in India is sparse.
Objective: Our study aims to compare and evaluate the treatment outcomes and treatment-related adverse events in newly diagnosed ITP pts treated with corticosteroids (Prednisolone/ Dexamethasone) in terms of initial response at 4 weeks (wks) and sustained response at 3 months from initiation of therapy respectively.
Methods: We enrolled 73 pts with new onset ITP, attending the outpatient department of AIIMS Rishikesh, between March 2019 to August 2020. Pre-treatment Complete Blood Count(CBC) Platelet count (PLT), Reticulocyte count, LDH, Liver and Renal Function Tests, PT, APTT, ANA, HIV, HBsAg, HCV, USG Abdomen, and Chest X-Ray were done. Bone marrow aspirate and biopsy (BM) was done in pts >50 yrs or to look for other hematological disorders. BM done in 19pts was normal. All pts had a pretreatment PLT <30 x 109/L. Children (age <12yrs) were treated only in the presence of bleeding. The severity of bleeding was graded as per the WHO bleeding score. Pts were randomized by block randomization and treated with Prednisolone or Dexamethasone. Prednisolone was administered at 1mg/kg/day for 4 wks (age >12yrs) and 2mg/kg/day for 2 wks (age < 12yrs) and tapered gradually over 2 months. Dexamethasone was administered at 40mg/day for 4 days/cycle, for 3 cycles at an interval of 14 days. Pts were followed up every wk for the first 4 wks and monthly thereafter for 3 months with CBC, PLT, and evaluated for any possible bleeding/complications. Response was defined as Complete response (CR) plt ≥ 100 x 109 /L. Response (R) plt ≥ 30 x 109/L and >2 fold increase in plt from baseline, no bleeding. No response (NR) plt < 30 x 109 /L or <2 fold increase in plt from baseline or bleeding. Loss of response (LR) plt < 30 x 109/L or bleeding, from CR or R previously.
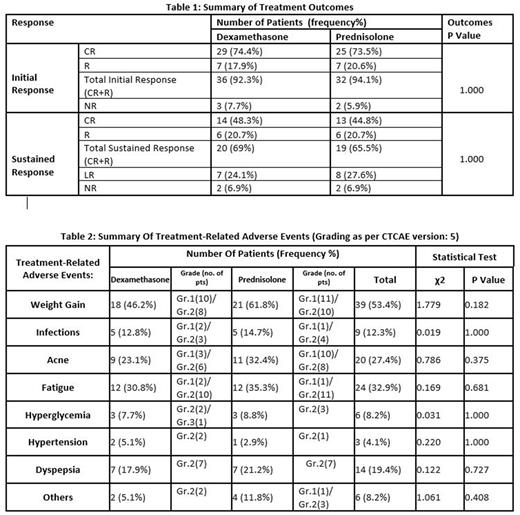
Results: Of the 73 pts evaluated, 23(32%) were males and 50 (68%) females. The median age was 29 (range 3 to 75years), of which 13 (18%) were pediatric pts (age <12 yrs). 10 (14%) pts had no bleeding at presentation, 63 pts (86%) presented with bleeding, of which 23 pts (45%) had mild bleeding grade 1 -2, 27pts (37%) had moderate bleeding grade 3, and 4 pts had severe bleeding grade 4 including 1 pt with intracerebral bleed. 39 (53%)pts received dexamethasone, and 34 (47%)pts received prednisolone. 68 out of 73 pts (94%) had an initial response at 4 weeks, 54pts (74%) attained CR, and 14(20%) had R. 5pts had NR in whom steroids were discontinued and second-line therapy was started. Sustained response was assessed at 3 months in 58 out of 73pts, 15 were lost to follow-up. An overall sustained response CR and R was seen in 39 pts (67%), 15 (26%) had loss of response LR and 4 (7%) had no response NR. The results are summarised in table 1. 57 (88%) out of 73pts had treatment-related adverse events (TRAE) and 16 (22%) had no TRAE. The TRAE observed in our study include weight gain, infections, acne, fatigue, hyperglycemia, hypertension, dyspepsia, and others (insomnia, proximal myopathy, & central serous retinopathy-CSR). 1pt had proximal myopathy and 1 pt developed CSR. The severity of TRAE was graded as per CTCAE. There was no grade 4 or 5 TRAE. All TRAE resolved spontaneously or with treatment. TRAE is summarised in table 2. No significant difference was noticed between the prednisolone or the dexamethasone group either in terms of treatment outcomes or treatment-related adverse events.
Conclusion: Our study shows comparable results between prednisolone and dexamethasone groups in initial and sustained response rates to first-line therapy in newly diagnosed ITP patients but a high incidence 88% of treatment-related adverse events in both groups. A shorter duration of steroids and affordable newer modalities of therapy in ITP is required to improve the overall well-being of ITP patients with lower middle income in India.
Disclosures: No relevant conflicts of interest to declare.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.