Abstract
Background: Patients with hemophilia A suffer from decreased bone mineral density. It is unknown whether the absence of factor VIII (FVIII) might play a direct causative role.
Aim: To investigate the effect of recombinant FVIII (rFVIII, simoctocog alfa) and von Willebrand factor (vWF) on osteoclastogenesis in a humanized two-dimensional (2D) cell culture model. Simoctocog alfa is a rFVIII product manufactured using a human cell line without chemical modification or protein fusion.
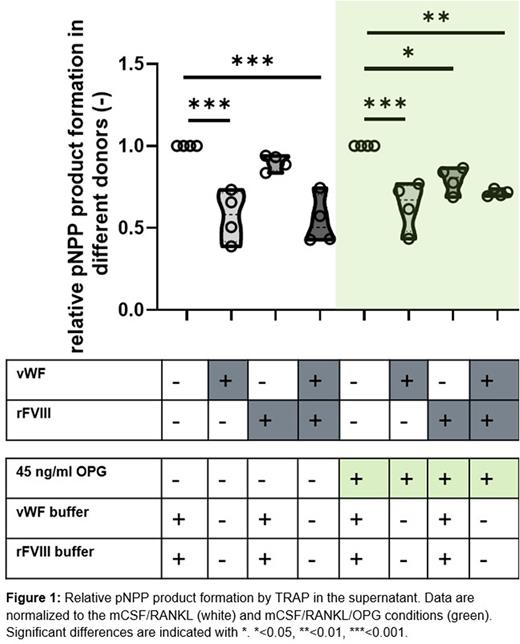
Methods: CD14+ monocytes from male donors were cultured for 14 days with 10% human serum under the influence of macrophage-colony stimulating factor (M-CSF) and receptor activator of nuclear factor kappa-B ligand (RANKL), in the presence and absence of osteoprotegerin (OPG). On days 0, 4, 7 and 11 cells were incubated with 10 IU/ml rFVIII, 10 IU/ml vWF or 10 IU/ml rFVIII/vWF. Tartrate-resistant acid phosphatase (TRAP) activity was measured in the supernatant on day 14 using a colorimetric assay with p-nitrophenylphosphate (pNPP) as substrate. Data were normalized to the condition with mCSF/RANKL alone, or mCSF/RANKL/OPG. Quantitative PCR of cathepsin K (CATK) and nuclear factor of activated T cells (NFATc) was performed.
Results: In the absence of OPG, osteoclastogenesis was significantly reduced by the addition of vWF (p<0.001) or the combination rFVIII/VWF (p<0.001). In the presence of OPG, addition of vWF (p<0.001), rFVIII (p<0.05), or the combination rFVIII/VWF (p<0.01) significantly reduced osteoclastogenesis. Similar results were obtained for analyses of CATK and NFATc gene expression.
Conclusion: We demonstrated an inhibitory effect of rFVIII, vWF and rFVIII/VWF on osteoclastogenesis in an in vitro model using human serum as opposed to the more commonly used fetal calf serum. As osteoclastogenesis has a high donor-to-donor variation, we are currently testing more donors to see if dependencies on age or blood type exist. These data provide a first insight into mechanisms underlying the reduced bone mass observed in patients with hemophilia A.
Disclosures
Kimenai:Octapharma AG: Other: Mrs Kimenai's employment is with the Balgrist Campus AG, which is not an ineligible company. As part of a contract between the Balgrist Campus AG and Octapharma AG, the salary of Mrs. Kimenai is compensated in full by Octapharma AG., Research Funding. Botter:Octapharma AG: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.