Abstract
Background: Eptacog beta (rFVIIa-jncw) (brand name SEVENFACT®) was approved by the U.S. Food and Drug Administration (FDA) in 2020 for the treatment of bleeding events (BEs) in individuals 12 years of age and older with hemophilia A or B (HAB) complicated by inhibitors. ATHN 16: Safety of Coagulation Factor VIIa (recombinant)-jncwforthe Treatment of Bleeding Events in Patients with Hemophilia A or B with Inhibitors was designed to collect real-world evidence on the safety of eptacog beta.
Objective: To evaluate the safety of eptacog beta when used to treat BEs in participants with HAB with inhibitors with or without FDA-approved prophylactic treatment.
Methods: ATHN 16 (NCT04647227) is a phase IV, United States-centric multicenter, open-label safety study enrolling participants with HAB with inhibitors aged 12 years and older, who are either on an FDA-approved prophylaxis and at risk of experiencing a breakthrough BE or who are not on prophylactic treatment and may need to control a BE.
Exclusion criteria include any bleeding disorder in addition to HAB, a known hypersensitivity to eptacog beta or rabbit proteins, or the inability to provide informed consent. The maximum study duration for any participant in the study will be up to 2 years from the time of enrollment.
Safety of eptacog beta is evaluated based on events included in the European Haemophilia Safety Surveillance (EUHASS) protocol, including allergic or other acute events, treatment-emergent side effects, transfusion-transmitted infections, inhibitor (FVII) development, thrombosis, cardiovascular events, malignancies, neurological events, and deaths.
After informed consent is obtained, demographics, bleeding disorder history, inhibitor history, baseline medical and surgical history are recorded for the baseline data. Each participant receives nine 75 µg/kg doses of eptacog beta supplied by the study funder. Eptacog beta is administered by the participant or by study staff at the time of a BE. The doses and duration of treatment are determined at the discretion of the treating physician. BE details including timing of BE, any treatments associated with the BE (including eptacog beta) and timing of resolution of the event, are collected as well as surgical procedures, EUHASS events, adverse events of special interest (AESIs), and serious adverse events (SAEs).
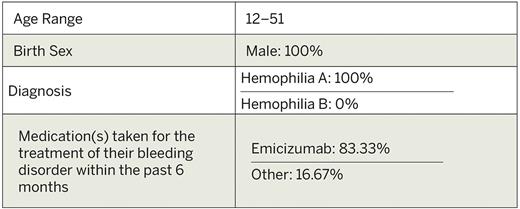
Results: At the time of abstract submission, ATHN 16, having received central IRB approval, is rolled out to ATHN-affiliated sites across the United States. The study is currently open to enrollment at 11 sites and an additional 21 sites are proceeding with start-up approvals. A total of seven participants have been enrolled. One BE, unrelated to study product, has been reported. One SAE, unrelated to study product, has been reported.
Conclusions: To date, a total of seven participants have enrolled in ATHN 16. One bleeding event has been treated with eptacog beta. The results will be updated, and all safety endpoints will be updated prior to the December 2022 meeting.
Disclosures
Chrisentery-Singleton:Pfizer: Consultancy, Honoraria, Research Funding; Kedrion: Consultancy, Honoraria; Genentech: Consultancy, Honoraria, Speakers Bureau; Bayer: Consultancy; CSL Behering: Consultancy, Honoraria, Speakers Bureau; Biomarin: Consultancy, Honoraria, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; Octapharma: Consultancy, Honoraria, Speakers Bureau; Hema Biologics: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; GBT: Consultancy, Honoraria, Speakers Bureau. Amos:Sanofi: Consultancy, Honoraria; Genetech: Consultancy, Honoraria. Bonzo:Employee of LFB: Current Employment. Escobar:Genentech: Honoraria; CSL Behring: Honoraria; Bayer: Honoraria; Takeda: Honoraria; Novo Nordisk: Honoraria; UniQure: Honoraria; Sanofi: Honoraria; Kedrion: Honoraria; Hemobiologics/LFB: Honoraria; The National Hemophilia Foundation: Honoraria; Pfizer: Honoraria; BioMarin: Honoraria. Giermasz:ATHN: Consultancy, Research Funding; LFB: Other: co-investigator in research funded by ; uniQure: Research Funding; Pfizer: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; Hema Biologics: Consultancy; Genentech: Consultancy, Speakers Bureau; Sanofi Genzyme: Consultancy, Speakers Bureau; BioMarin: Consultancy, Research Funding, Speakers Bureau; Sangamo: Research Funding; Adrenas: Consultancy. Lagrue:Employee of LFB: Current Employment. Nasr:Hema Biologics: Consultancy. Recht:Catalyst Biosciences, CSL Behring, Genentech, Grifols, Hema Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, uniQure: Consultancy; Bayer, Biomarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, uniQure: Research Funding; Foundation for Women and Girls with Blood Disorders; Partners in Bleeding Disorders: Thrombosis and Hemostasis Societies of North America: Membership on an entity's Board of Directors or advisory committees; Oregon Health & Science University: Ended employment in the past 24 months; American Thrombosis and Hemostasis Network; Yale University School of Medicine: Current Employment. Sullivan:Octapharma: Consultancy; Biomarin: Consultancy, Speakers Bureau; Pfizer: Consultancy; Genentech: Consultancy; Bayer: Consultancy; Mississippi Center for Advanced Medicine: Current Employment; Make-A-Wish Mississippi: Membership on an entity's Board of Directors or advisory committees. Quon:Novo Nordisk: Consultancy, Honoraria, Other: travel support , Speakers Bureau; Bayer: Consultancy; Octapharma: Consultancy; Takeda: Consultancy, Honoraria, Other: Clinical trial investigator, travel support, Speakers Bureau; BioMarin Pharmaceutical Inc.: Consultancy, Honoraria, Other: clinical trial investigator, travel support , Speakers Bureau; Roche/Genentech: Consultancy, Honoraria, Other: Clinical trial investigator, travel support , Speakers Bureau; Sanofi: Consultancy, Honoraria, Other: Clinical trial investigator, travel support , Speakers Bureau; UniQure: Other: Clinical trial investigator. Reding:Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Biomarin: Consultancy, Honoraria, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Honoraria, Speakers Bureau; Hema Biologics: Consultancy, Honoraria; Novo Nordisk: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.