Abstract
Introduction:
PET/CT (Positron Emission Tomography/Computed Tomography) scans are the preferred modality of treatment response monitoring and surveillance in patients with diffuse large B-cell lymphoma (DLBCL). However, the interpretation of PET/CT results can be challenging due to low sensitivity of 18F-fluorodeoxyglucose (FDG) tracers, leading to high rates of false positivity and false negativity at the interim and end-of-treatment scans. Circulating tumor DNA (ctDNA) has been demonstrated as an independent prognostic biomarker across numerous cancer types including lymphoma. To date, studies examining the use of ctDNA have mostly employed targeted gene panels, which may not appropriately capture the genetic heterogeneity of the disease. Here, we examined the feasibility of utilizing personalized and tumor-informed ctDNA testing in patients with DLBCL.
Methods:
In a real-world study, 51 patients diagnosed with systemic DLBCL were enrolled from September 20, 2020 to May 5, 2022. ctDNA analysis was conducted using a personalized, tumor-informed assay designed to detect the top 16 clonal mutations identified in a patient's tissue specimen (SignateraTM bespoke mPCR NGS assay). For newly diagnosed and relapsed/refractory patients, blood samples were collected and analyzed at diagnosis (for newly diagnosed DLBCL), prior to each cycle of therapy, and every 3 months thereafter during surveillance. Our primary objective was to determine the feasibility of developing a bespoke ctDNA assay for individual patients and evaluate concordance rates between ctDNA levels at baseline with the presence of active disease on PET/CT scans. Secondary objectives were to evaluate ctDNA kinetics during treatment and investigate correlation with PET/CT results. Exploratory analyses included correlation of baseline ctDNA levels at diagnosis with high-risk features, such as double/triple hit disease, Ki67, and revised International Prognostic Index (R-IPI), as well as survival outcomes. Clinical follow-up is available for all patients and longitudinal ctDNA assessment is ongoing.
Results:
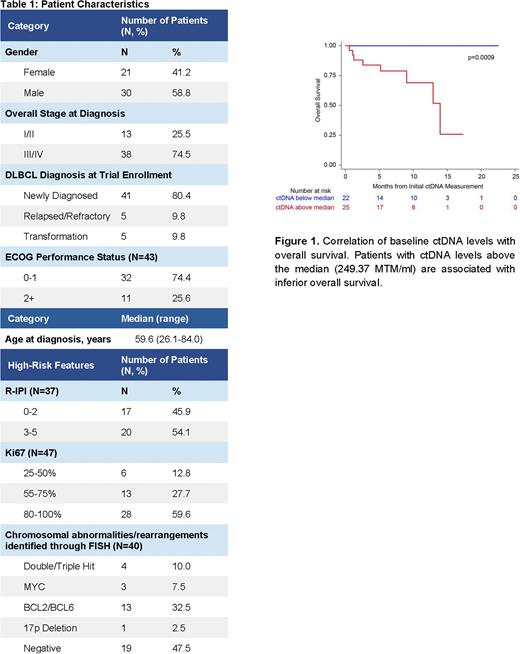
For all patients enrolled in the study, staging information and patient characteristics are outlined in Table 1. Of the 51 patients analyzed for ctDNA, a personalized assay with 16 tumor-specific mutations was successfully designed for 49 patients (96.1%). Of those, 47 were starting upfront or second line therapy and 2 were being monitored during surveillance. Prior to treatment initiation, ctDNA was detected in 97.9% (46/47) of patients with levels ranging from 0.1-5957.8 mean tumor molecules (MTM)/ml of plasma. Of the 46 ctDNA-positive patients, longitudinal assessment of ctDNA revealed concordance with radiologic findings at the restaging PET scan. ctDNA levels at baseline were prognostic of patient outcomes. Patients with ctDNA levels above the median (249.37 MTM/ml) had inferior overall survival compared to patients with ctDNA levels below the median (p=0.0009) (Figure 1). Lastly, we plan to present analyses on the correlation of ctDNA levels with clinical risk features and ctDNA dynamics with patient outcomes.
Conclusion:
Measurement of ctDNA with a personalized and tumor-informed assay is feasible in patients with DLBCL, with a baseline detection rate of 97.9%. Baseline ctDNA levels were prognostic with higher MTM/ml correlating with inferior OS. Our results suggest that ctDNA is a useful biomarker and should be considered when evaluating patient response to treatment. Taken together, longitudinal ctDNA monitoring is feasible in DLBCL and could provide further insights into disease progression. Additional follow-up and clinical studies are needed to evaluate the utility of ctDNA testing for clinical decision making in this setting.
Disclosures
Narkhede:EUSA pharmaceuticals;: Research Funding; Gilead/Forty-seven: Research Funding; Gilead: Research Funding; Roche: Research Funding; Genetech: Research Funding; Genmab: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Seagen Inc.: Research Funding. Tomassetti:Principia: Research Funding; Merck: Research Funding; SeaGene: Research Funding; Rigel: Research Funding; Beigene: Research Funding; Novartis: Research Funding. Widden:Natera, Inc.: Current Employment, Current equity holder in publicly-traded company. Benrud:Natera, Inc.: Current Employment, Current equity holder in publicly-traded company. Rathore:Natera, Inc.: Current Employment, Current equity holder in publicly-traded company. Kalashnikova:Natera, Inc.: Current Employment, Current equity holder in publicly-traded company. Koyen Malashevich:Natera, Inc.: Current Employment, Current equity holder in publicly-traded company. Liu:Natera, Inc.: Current Employment, Current equity holder in publicly-traded company. Aleshin:Natera, Inc.: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.