Abstract
Introduction: Nodal peripheral T-cell lymphomas (nPTCL) constitute a heterogeneous group of rare malignancies with aggressive biological behavior and poor prognosis. Epigenetic phenomena involving genes that control DNA-methylation and histone deacetylation, such as IDH2, DNMT3A, TET2 and RhoA, play a central role in their pathogenesis. Tumor mutation burden (TMB) has emerged as a prognostic biomarker in numerous solid neoplasms. Furthermore, TMB reflects the process of clonal evolution, with progressive acquisition of additional molecular-genetic abnormalities. Recent studies were capable to establish a relationship between TMB and response to anti-cancer treatments. In 2021 Fachi et cols. established a correlation between high TMB in epigenetic regulatory genes and higher response rates to 5-azacytidine and romidepsin in nPTCL. However, the potential prognostic impact of epigenetic TMB remains unknown in nPTCL. Based on this premise, here we aimed, in a pioneering way, to search for a correlation between epigenetic high TMB and adverse prognosis in nPTCL.
Methods: This is a retrospective, observational and single-center study, involving 59 patients with nPTCL diagnosed and treated at the Department of Hematology, University of São Paulo, Brazil, from January 2000 to December 2019. Formalin-fixed paraffin-embedded (FFPE) diagnostic tumor samples were submitted to Sanger sequencing with a target-panel, involving IDH2, DNMT3A, TET2 and RhoA genes to search for recurrent mutations. Only non-synonymous mutations were considered for prognostic association. Survival curves were constructed using the Kaplan-Meier method and the Log-Rank test was used to establish a relationship between TMB and outcomes. Results were presented as hazard ratio (HR) and 95% confidence interval (95% CI), and a p-value < 0.10 was considered statistically significant.
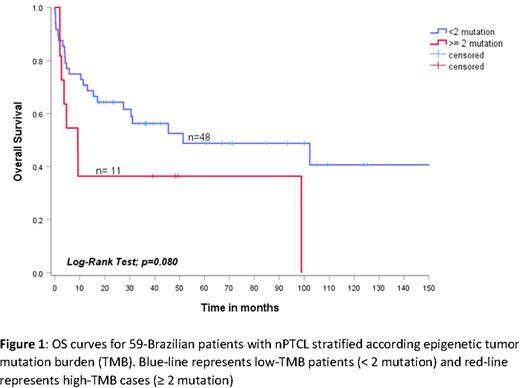
Results: The median age was 50 years (IqR 38-61), and 57.6% (34/59) were male. Thirty-nine percent (23/59) had bulky disease ≥ 7 cm, 83% (49/59) had B-symptoms, 94.9% (56/59) had advanced stage III/IV, 27.1% (16/59) had ECOG ≥ 2, 27.1% (16/59) had ≥ 2 extranodal sites involved by lymphoma, and 55.9% (33/59) had intermediate-high/high-risk IPI. Seventy-six percent (45/59) received up-front therapy with CHOP-like regimens (CHOP or CHOEP), 23.7% (14/59) experienced radiotherapy, and 33.3% (20/59) were consolidated with ASCT. With a median follow-up of 3.7 years (95% CI: 0.9-12.4), the estimated 2-year OS and PFS were 57.1% (95% CI: 45.5-70.4) and 49.2% (95% CI: 34.0-59.2), respectively. Missense, non-sense or frameshift mutations in the IDH2 gene were observed in 3.3% (2/59) of cases, DNMT3A in 3.3% (2/59), RhoA in 23.7% (14/59) and TET2 in 37.2% (22/59). Among the 59 patients included, 23/59 (38.9%) had no mutations in the target-genes, 25/59 (42.4%) had 1 mutation, and 11/59 (18.6%) had two or more mutations. Therefore, the cases were categorized into low TMB (< 2 mutations) [n=48/59 - 81.3%] or high TMB (≥ 2 mutations) [n=11/59 - 18.7%]. The median overall survival was 51.4 months (95% CI: 0-124.1) for low TMB and only 3.1 months (95% CI: 3.1-15.2) for high TMB, p=0.08. The estimated 2-year OS was 64.3% (95% CI: 50.6-78.0) for low TMB and 36.4% (95% CI: 8.0-65.0) for high TMB [Figure 1]. Similarly, median progression-free survival was 13.3 months (95% CI: 0-29.4) for low TMB and 6.3 months (95% CI: 0.9-11.6) for high TMB, p=0.319. The estimated 2-year PFS was 46.1% (95% CI: 20.0-72.1) for low TMB and 25.0% (95% CI: 17.5-67.5) for high TMB.
Conclusion: In a pioneering way, we demonstrated that the high epigenectic tumor mutation burden (TMB), defined by the presence of 2 or more mutations involving epigenetic regulatory genes, was associated with decreased overall survival in Brazilian patients with nodal PTCL. Although preliminary, these data support the potential impact of epigenetic TMB as a prognostic biomarker in nPTCL.
Disclosures
Rocha:Amgen: Research Funding; Takeda: Speakers Bureau; Pfizer: Speakers Bureau; Amgen: Speakers Bureau. Pereira:Janssen: Research Funding; Zodiac: Honoraria; Astrazeneca: Honoraria, Research Funding; Bayer: Research Funding; Celltrion: Research Funding; Sandoz: Honoraria, Research Funding; Eusa: Honoraria; Takeda: Honoraria; Libbs Farmacêutica: Honoraria, Research Funding; Roche: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.