Abstract
Background: Diffuse Large B-cell Lymphoma (DLBCL) is the most common lymphoma subtype. The standard first line treatment is rituximab, cyclophosphamide, adriamycin, oncovine and prednisone (RCHOP). This regimen, will cure roughly 80% of low risk and 40% of high risk patients. There have been several attempts to improve the cure rate of patients in complete response (CR) after RCHOP with maintenance therapy, amongst them with rituximab and lenalidomide but neither one was successful. Nivolumab is an anti-PD1 antibody that was found to be effective in a proportion of patients with relapsed DLBCL. In this phase 2 trial we aimed to test the efficacy and toxicity of nivolumab maintenance therapy in first CR in patients with DLBCL, in attempt to eradicate minimal residual disease and prevent relapse.
Methods: Patients with IPI ≥ 2 who completed 6 cycles of rituximab and anthracycline based regimens and achieved complete response (CR) per PET scan according to the Lugano criteria were eligible. Individuals with active autoimmune disorder, CNS disease or any other clinically significant disease were excluded. All participants were included in the study up to 12 weeks post their end of therapy PET scan. Treatment was given every 2 weeks in a dose of 240 mg for 8 cycles and then 480mg every 4 weeks for up to 2 years or 30 cycles (which ever occurred first). We aimed to recruit 52 patients but due to slow accrual the study was terminated early.
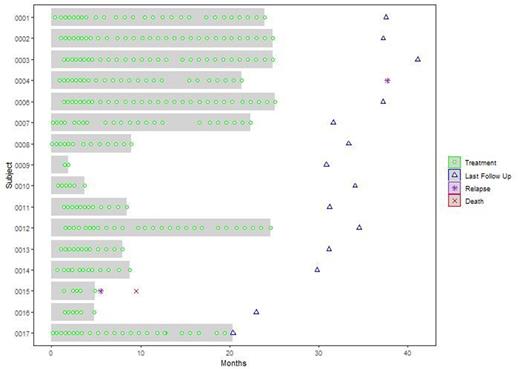
Results: Sixteen patients, 10 male and 6 female were treated between December 2018 and June 2022. Median age was 67.5 years (51-74). Fourteen patients had DLBCL NOS, 2 had double /triple hit lymphoma. All patients received RCHOP as their first line. Seven patients (43.75%) completed the treatment period, 8 (50%) did not and one patient is still in treatment. Of the 8 patients who did not complete the treatment 7 stopped due to adverse events and 1 due to disease progression. Altogether 2 patients relapsed (12.5%), one during the study period and another after completion (figure 1). All patients received at least 2 cycles of treatment, and only 12% (n=2) received 30 cycles. All patients had at least one adverse event (AE) and 12 patients had grade 3 or more AE's. Of the 12 patients, 7 (43.75%) had grade 3 and 5 (31.25%) had grade 4 AE as their most severe event. We documented 8 serious adverse events (SAE's) in 5 patients (31.25%). The SAE's were: colitis (3 patients), pancreatitis (3 patients), subdural hematoma (1 patient), thermal burn (1 patient) and adenocarcinoma of colon (1 patient). Two of the SAE's were drug related (colitis) and one was considered possibly related (pancreatitis), all occurred in the same patient. Three (18.75%) patients had anemia, in one them it was hemolytic, 4 patients (25%) had total of 6 events of neutropenia, and 5 of them were severe. Three patients had treatment interruptions due to toxicity and 11(69%) required treatment with steroids in a total of 22 events. One patient deceased due to disease progression. No patient died due to AE's.
Conclusions: Nivolumab maintenance in this small cohort of DLBCL patients with CR after RCHOP showed a signal of lower than the expected relapse rate. However, the unexpected high rate of drug related AE's caused many treatment interruptions and prevented the completion of treatment in 7/16 (44%) patients. Overall, nivolumab maintenance in DLBCL patients achieving CR after 1st line therapy may be effective, but the relatively high rate of toxicity may preclude its application. Based on the Hodgkin Lymphoma trials we did not anticipate such a high toxicity rate. We suspect this discrepancy is related to the previous treatment with rituximab.
Disclosures
Benjamini:AbbVie,Janssen,Astrazeneca: Consultancy. Avigdor:Takeda, Gilead, Novartis, Roche, BMS: Consultancy; AbbVie: Honoraria.
OffLabel Disclosure:
Nivolumab in DLBCL in first CR.
Author notes
Asterisk with author names denotes non-ASH members.