Abstract
Introduction: Ever since the availability of BCR-ABL1 tyrosine kinase inhibitors (TKIs) as treatment for chronic myeloid leukemia (CML), life expectancy of patients has dramatically improved, approaching that of the overall population. Several clinical trials have demonstrated the possibility of discontinuing TKI. However, the information on the safety of long-term TKI cessation is still limited.
Methods: We carried out a descriptive, retrospective and single-center study, including 56 patients with CML who discontinued TKI treatment between January 2006 and July 2022. We defined strict molecular relapse as loss of MR4 (BCR-ABL1 International Scale (IS) ≤ 0,01% or undetectable BCR-ABL1 in samples with ≥10.000 transcripts), loose molecular relapse as loss of major molecular response (MMR) (BCR-ABL1 IS ≤ 0,1% in samples with ≥10.000 transcripts) and deep molecular response (DMR) as response greater than or equal to MR4. Progression-free survival (PFS) was estimated by the method of Kaplan-Meier and defined as the time from TKI discontinuation to the date of first event (loss of DMR or death).
Results: We included 56 patients (table 1). Median age at time of stopping TKI treatment was 60,3 (50,7-75,8) years and 35 (62,5%) were male. 44 patients had received 1 line of TKI treatment, 7 two lines, 2 three lines and 3 four lines. The previous TKI treatment consisted of imatinib (n=34), nilotinib (n=6), dasatinib (n=34), two lines of imatinib because of pregnancy (n=1), imatinib dasatinib (n=3), imatinib nilotinib (n=1), dasatinib imatinib (n=1), dasatinib ponatinib (n=1), imatinib dasatinib nilotinib (n=1), nilotinib dasatinib nilotinib (n=1), imatinib dasatinib imatinib dasatinib (n=1), imatinib nilotinib dasatinib dasatinib+asciminib (n=1), imatinib nilotinib dasatinib nilotinib (n=1). Duration of imatinib treatment was 121,5 (100-172,8) months, dasatinib 74,4 (29-86,1), nilotinib 34,3 (19,1-45,6), ponatinib 49 and asciminib 20,7. The reasons for switching from first to second line were intolerance (n=7, 58,3%), resistance to any TKI (n=4, 33,3%) and pregnancy (n=1, 8,3%). From second to third line was intolerance (n=5, 100%).
Duration of DMR before TKI discontinuation was 87,6 (49,2-116,4) months. The last molecular responses before TKI cessation were RM5 (n=18, 32,1%), RM4.5 (n=28, 50%), RM4 (n=9, 16%) y MMR (n=1, 1,8%). Reasons for TKI discontinuation were the presence of side effects (n=28, 50%), an attempt to achieve a treatment-free remission (TFR) in patients with a DMR (n=16, 28,6%), patient preference (n=10, 17,8%) and pregnancy (n=2, 3,6%).
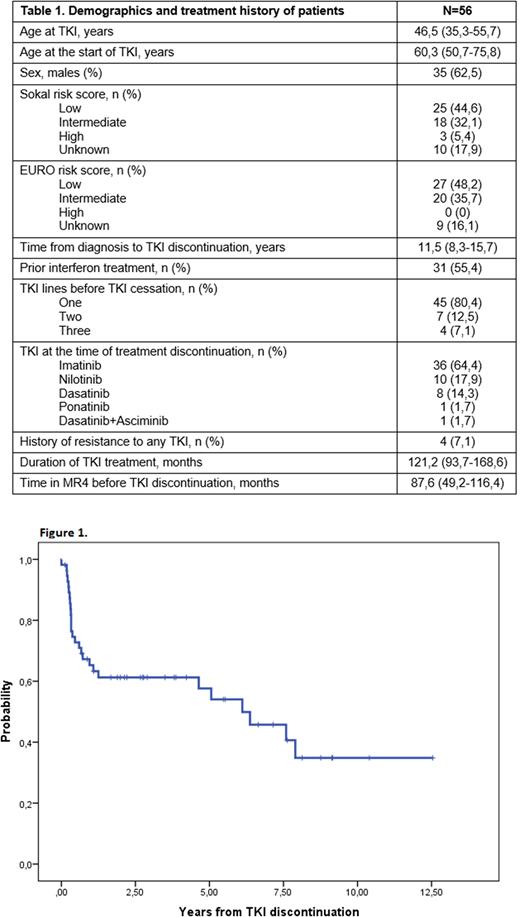
After TKI discontinuation, 34 (60,7%) patients have maintained DMR for a median of 4,22 (2,12-7,14) years, of which six more than 8 years being the maximum follow-up of 12,6 years. 5 patients had died while DMR due to CML-unrelated causes. The probability of PFS at 1 year and 12,6 years was 65,3% (95% CI: 58,6%-71,7%) and 34,8% (95% CI: 25%-44,1%) respectively (Figure 1).
22 (39,28%) patients had strict molecular relapse with a median of 3,9 (3,2-7,8) months, of which 12 (21,4%) were loose molecular relapses. Loss of DMR occurred most frequently in the first 6 months after TKI discontinuation (n=15, 68,1%). The last CMR loss was at 95 months. 18 patients restarted treatment, 2 died due to CML-unrelated causes, 1 maintained RMM without treatment due to intolerance and 1 stopped treatment due to personal preference. At the time of restarting treatment, the BCR-ABL1 IS was ≤10% (n=3), ≤1% (n=7), ≤0,1% (n=7) and ≤0,01% (n=1). After restarting treatment, 18 (100%) patients had MMR, of whom 16 (80%) had DMR. Median time to regaining DMR after resuming TKI therapy was 3 (0,9-3,05) months.
A second attempt at TFR was made in 5 patients. The median duration of TKI therapy after restarting the treatment and the second discontinuation was 60,2 (40,2-63) months. 4 patients maintained CMR with a follow-up of 11 (7,2-24,4) months, and one patient died while DMR for reasons unrelated to CML.
Adverse effects improved in most patients. TKI withdrawal syndrome was noticed in 12 (21,4%) patients.
Conclusion: In our study, the PFS at 12,6 years was 34,8%. Including the patients who have had a second discontinuation of TKI, 39 (69,6%) patients remain in DMR off-treatment, with a median follow-up of 49,1 (26,7-85,7) months. 6 patients had a follow-up of more than 8 years without treatment, which is still ongoing. These data confirm the applicability and safety of TKI discontinuation.
Disclosures
Loscertales:Lilly: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Speakers Bureau; Gilead: Speakers Bureau; Beigene: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.