Abstract
WHO classifies Histiocytic Sarcoma (HS) and Interdigitating Dendritic Cell Sarcoma (IDCS) as separate, unrelated, entities under histiocytic/dendritic cell neoplasms. We do not fully understand the pathogenesis of HS and IDCS, yet both have been shown to transdifferentiate from B cell non-Hodgkin lymphomas. Diagnosis is challenging and requires application of a wide variety of immunohistochemical studies, flow cytometry and molecular analysis. We present a case of HS which evolved from CLL/SLL and presented as a diagnostic challenge due to unusual features overlapping with what is typically seen in IDCS.
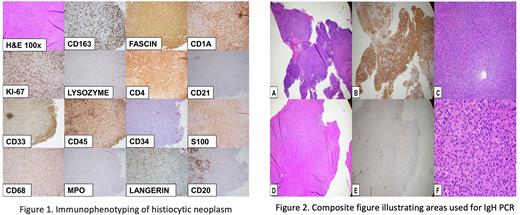
An 82-year-old male with a history of CLL/SLL presented with right infiltrative tonsillar mass and cervical lymphadenopathy. Biopsy of the lymph node revealed two abnormal populations of cells : Twenty percent of cells were consistent with CD20+ kappa surface light chain restricted CLL/SLL cells. Eighty percent of cells were histiocytic cells with an abnormal immunophenotype showing expression of CD4, CD43, fascin, HLA-DR, PU.1, (weak) S100 and CD1a without CD163, lysozyme, CD34, CD117, CD21, CD23 or CD14. Expert consultation favored the diagnosis of HS with transdifferentiation from CLL/SLL. PCR for IgH gene rearrangement, performed on separate HS-rich and CLL-rich areas of the biopsy detected identical clonal peaks. Next generation sequencing detected pathogenic KRAS mutations (p.G12D, p.A146P) and likely pathogenic BCOR (p.K839fs*17) and FBXW7 (p.R465S) mutations. While HS was the favored diagnosis, some immunophenotypic features were unusual, including diffuse fascin immunoreactivity, weak S100, and CD1a immunoreactivity which raised the possibility of so-called IDCS.
While clonal evolution of HS and IDCS from B cell Non-Hodgkin lymphomas has been reported, there is a paucity of literature on these entities. We suggest that there may be a pathogenic relationship between these two neoplasms that has yet to be elucidated due to their rarity and diagnostic challenge. Few clinical laboratories can perform the breadth of markers required for diagnosis. Both neoplasms have varied clinical presentations with nodal or extranodal involvement and portend poor prognosis. Review of the literature shows significant overlap in immunophenotypic and genomic characteristics of IDCS and HS, suggesting they may be related manifestations of the same neoplasm. A case report has described the initial presentation of IDCS that subsequently developed into HS, although sampling may have been an issue. We identified 11 cases, including our case in the literature presenting with positive histiocytic and dendritic cell markers referred as hybrid dendritic-histiocytic sarcomas or histiocytic sarcoma with interdigitating dendritic cell differentiation. Immunohistochemical analysis of these cases showed positivity rates of S100 (100%), lysozyme (82%) and CD1a (18%). One case in the literature described fascin immunoreactivity, as was observed in our case. Four of six cases had CD163 immunoreactivity. A hybrid neoplasm with overlapping features of follicular and interdigitating dendritic cells has also been reported, further confounding our understanding of these neoplasms. Large studies analyzing genomic features of these neoplasms have shown recurrent mutations in RAS-MAPK pathway, as was seen in our case. Further studies of the relationship between HS, IDCS should include gene expression profiling of these entities which may also yield therapy targets.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.