Abstract
Introduction
Inherent complex pathophysiology of myelodysplastic syndrome (MDS) along with its higher occurrences in elderly population often limits available treatment options. In Japan, azacitidine (AZA) is the only first-line (1L) treatment, which is not cytarabine-based, available for patients (pts) with high-risk MDS (HR-MDS) ineligible for hematopoietic stem cell transplantation (HSCT). Prior autoimmune diseases have been associated with susceptibility of MDS. However, durability of AZA treatment and data on MDS-specific comorbidities, especially immune-driven events are currently lacking in Japanese pts. This study aimed to answer clinical questions on AZA real-world treatment outcomes in Japan, the epidemiology of autoimmune diseases in this specific pt cohort, as well as treatment cost.
Methods
This was a retrospective, longitudinal study utilizing hospital-based administrative claims for MDS patients in the Medical Data Vision (MDV) database in Japan. Data on pt demographics, clinical characteristics, treatments, procedures and hospitalization records were collected. Adult pts with first a confirmed MDS diagnosis either as an inpatient or twice in outpatient setting during the identification period (1October 2008 - 30 September 2017), who had a bone marrow biopsy in ±3 months from index date and at least 1 MDS-related treatment post-index date, were included in this real-world study. Pts with confirmed acute myeloid leukemia (AML) diagnosis or who had any history of HSCT, graft-versus-host-disease or transplant-related disorder/complications were excluded. As IPSS/R scoring is unavailable in MDV, pts were classified as HR-MDS if they had either AZA treatment or HSCT within 6 months post-index date, or pancytopenia diagnosis within 6 months post-index date and initiated AZA within 12 months.
Results
Of 6204 pts who met the eligibility criteria (main cohort), 2138 pts were assigned as HR-MDS. The median age of pts in the main cohort was 74.0 years (range: 18.0 - 100.0 years, ≥60 years old: 87.8%) vs 73.0 years (range: 20.0 - 95.0 years; ≥60 years old: 88.6%) in the HR-MDS cohort. Mean Charlson Comorbidity Index was 1.7 (± 1.66) and 1.6 (± 1.47) for the main cohort and HR-MDS cohort respectively. The proportion of transfusion-dependent pts in the main cohort was 9.4% vs 7.1% of patients in the HR-MDS cohort.
Among 2138 pts with HR-MDS, 251 pts reported having concomitant autoimmune disease with rheumatoid arthritis (32.3%), glomerulonephritis (26.7%) and seronegative arthritis (23.1%) being the most common.
More pts in the main cohort (53.2%) compared to pts with HR-MDS (38.9%) received anti-anemic drugs, G-CSF and iron chelators as supportive therapy at 1L. Compared to 39.5% pts in main cohort, 88.3% pts with HR-MDS received antineoplastic agents at 1L therapy. In HR-MDS cohort, 2078 pts received AZA in different cycles (1-3 cycles: 40.5%, 4-6 cycles: 21.1%, 7-9 cycles: 11.5%, >9 cycles: 26.9%) but only 121 pts received intensive chemotherapy (1 cycle: 90.1%, 2 cycles: 9.1%, >2 cycles: 0.8%).
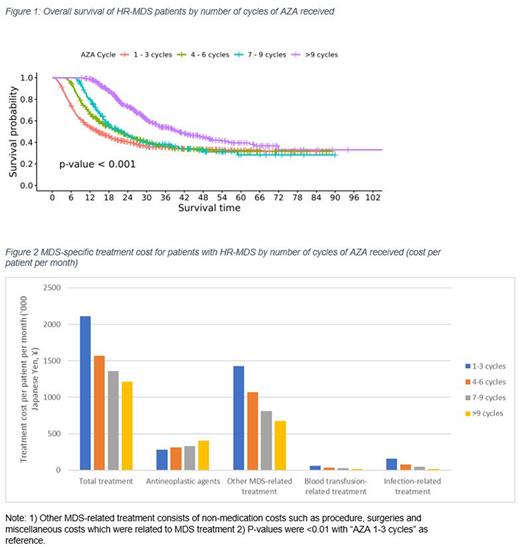
Median duration of MDS-specific treatment for patients with HR-MDS was 9.8 months. Significantly higher median OS (P<0.001) was achieved in pts with HR-MDS who received more cycles of AZA (Fig. 1: 1-3 cycles: 14.6 months, 4-6 cycles: 21.4 months, 7-9 cycles: 22.8 months, >9 cycles 40.8 months). After controlling for death as a competing risk, the cumulative incidence function (CIF) of HSCT and AML transformation was not affected by the number of cycles of AZA received. Although more cycles of AZA treatment increased the total treatment cost, median cost per pt per month was inversely affected with the lowest cost for pts receiving >9 cycles (Fig. 2).
Conclusion
Our findings based on the real-world data analysis in Japanese pts follow the similar trend of elderly pts with HR-MDS mostly receiving AZA as 1L treatment. Despite higher median OS being associated with higher number of AZA cycles, only ~27% pts with HR-MDS received >9 cycles of AZA. Autoimmune disease was reported by 11.7% of pts with HR-MDS. Although treatment cost increased with number of cycles of AZA received, median cost per pt per month was lowest in patients receiving >9 cycles. The cost per pt per month for other MDS-related treatment as well as costs for blood transfusions and infection related treatments were lower as increasing cycles of AZA were given which contributed to lower cost per pt per month.
Disclosures
Shioi:Novartis Pharma NPKK: Current Employment. Ooi:Novartis Corporation Malaysia Sdn Bhd: Current Employment. Tay:Novartis Corporation Malaysia Sdn Bhd: Current Employment. Wong:Novartis Pharma NPKK: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.