Abstract
Introduction: Multiple Myeloma (MM) remains incurable despite the advances made with the drugs such as proteasome inhibitors, immunomodulatory agents, antibodies, and hemopoietic stem cell transplantation (HSCT). B-cell Maturation Antigen (BCMA) is a surface receptor which can be targeted by Antibody-drug conjugates, Chimeric antigen receptor T cells (CAR-T), and Bispecific T cell engager (Bi-TE), these therapies have shown high response rates up to 70-100% in relapse-refractory MM (RRMM) (Tai et al., 2019). Despite the promising outcome, there are various adverse reactions associated with BCMA. In this focused review, we will discuss how to manage these complications.
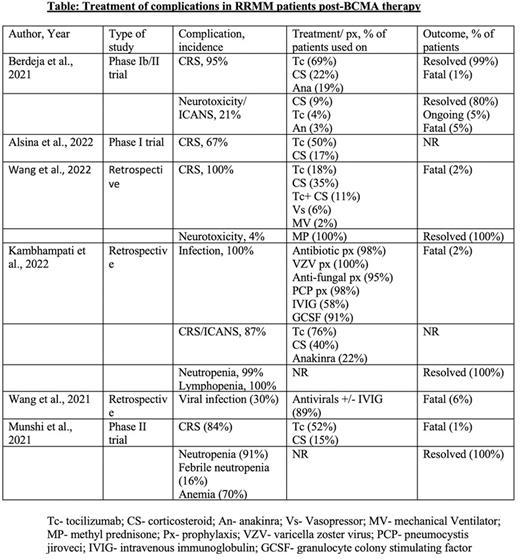
Method: We have performed literature search from PubMed and Google Scholar with the MeSH terms and keywords 'multiple myeloma', 'BCMA', 'infections', 'CRS', 'Cytopenia', 'ADC', 'CAR-T', and 'Bi-TE'. After reviewing about 150 articles, we have included the original articles in which the complication after BCMA therapy and its management were documented.
Result:
CRS and Immune effector cell-associated neurotoxicity syndrome (ICANS): CRS is the most common complication post-BCMA therapy, with the incidence found to be 80-100% after CAR-T cell infusion (Wang et al., 2022). Severity may vary from mild self-limiting illness to hemodynamic instability (Grade 1-IV) requiring vasopressor and ventilatory support. The cytokines mostly responsible is Interleukin-6 (IL-6), however IL-1, IL-2, IL-10, GM-CSF could also be found associated with it. Due to symptom overlap, infectious etiology should be ruled out before treating CRS. Tocilizumab is FDA approved for life-threatening CRS in patient aged two years or above, it is a monoclonal antibody that targets both membrane bound and soluble form of IL-6 receptor and competes with IL-6 to bind it (Le et al., 2018). Steroid is used in CRS refractory to tocilizumab or being administered concomitantly for high grade CRS or CRS associated with neurotoxicity. Siltuximab which binds to site I of IL-6 receptor and prevents IL-6 binding to it, is not FDA approved, however used in selective tocilizumab resistance cases or as an alternative therapy (Riegler et al., 2019). Anakinra is an IL-1 receptor antagonist, which was found to improve CRS in association with tocilizumab when compared to tocilizumab alone (Jatiani et al., 2020). Lenzilumab, is a monoclonal antibody that neutralizes granulocyte-monocyte colony stimulating factor (GM CSF), was also used in preclinical studies and found to be lessening the severity of CRS without dampening CAR-T cell function (Sterner et al., 2019).
Infections: The factors which increase the risk of infection in MM patients post-BCMA therapy are >3 prior lines of therapy, B-cell aplasia, infections 30-days before CAR-T, and post CAR-T lymphopenia. To prevent infection, it is recommended to use antibacterial prophylaxis for neutropenia, to be continued for at least a month after CAR-T. Also, it is recommended to use VZV and PCP prophylaxis for 6 months and 3 months respectively (Kambhampati et al., 2022). GM-CSF support can be considered to keep absolute neutrophil count (ANC) >1000 cells/mm3 but weigh risk vs benefits as GM-CSF can increase CRS in theory. Regular monitoring of viral load and antigens are also recommended.
Cytopenia: The mechanisms involved in cytopenia(s) are prior cytotoxic therapy, chemotherapy causing lymphodepletion, HSCT, or development of MDS. The mainstay of management for cytopenia(s) are supportive management with blood product transfusion to keep parameters above target, G-CSF, prophylactic anti-bacterial and anti-fungal agents in severe neutropenia. In most cases cytopenia would improve over a period of 12-months.
Conclusion: Given the recent use of BCMA therapy in RRMM, there is no standard guideline on how to manage the complications associated with it. Larger studies with longer follow up needs to be conducted, to address the safety of the therapy for better patient outcome
Disclosures
Anwer:Allogene Therapeutics: Research Funding; Janssen: Consultancy; BMS: Consultancy, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.