Abstract
BACKGROUND. Gaucher Disease (GD), an autosomal recessive metabolic disorder due to glucocerebrosidase deficit, includes three clinical phenotypes. GD1 is classically considered as a non-neuronopathic form, in contrast to the GD2 and GD3 forms. The protective role of N370S mutation against neurological impairment has been hypothesized in GD1 patients (pts). However, signs of parkinsonism and/or cognitive impairment and/or behavioural alterations have also been observed in GD1 pts with N370S mutation. Neuroimaging studies indicates that human intellectual ability is related to the brain structure, including cortical thickness (CT) and cortical gyrification (CG), that allows greater cognitive functionality. The prospective SENOPRO_GAUCHER study was planned to evaluate in depth the neurological and neuropsychiatric aspects in GD1 pts, using clinical and instrumental investigations.
AIMS. Data from the clinical and instrumental investigations were analyzed in order to a) evaluate the morphological aspects of the cerebral cortex of GD1 pts; b) compare morphological findings in GD1 pts with healthy control subjects (HCs); c) correlate cortical morphological abnormalities, genotypes, neuropsychological and neurological manifestations in GD1 pts.
MATHERIALS AND METHODS. Nineteen GD1 pts, aged >12 years (yrs) at the evaluation, were enrolled in the SENOPRO_GAUCHER study. Seventeen of these pts, as well as HCs, matched for age, sex, and education level, underwent a T1-weighted 3Tesla Magnetic Resonance Imaging (MRI) scan to evaluate brain morphometry such as CT and CG in each of the 360 evaluated brain regions (Human Connectome Project parcellation). Pts and HCs had previously undergone neurological and psychological evaluations, including motor parkinsonisms and mental status analysis.
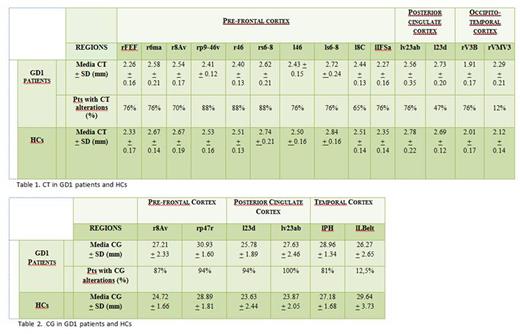
RESULTS. All but one of the 17 GD1 pts (M: 5; F: 12; median age 51 yrs) were heterozygous for N370S mutation. Detailed results of the 3T MRI are showed in Table 1 and 2. Thirteen regions of the prefrontal, posterior cingulate and parietal cortex corresponding to the functional domains of the working memory, attention, movement, language, and sensory integration were significantly reduced in CT in 16/17 N370S heterozygous GD1 pts than HCs (p< .05, uncorrected). CT in 5/14 prefrontal and temporo-occipital cortical areas was found lower in 4 pts with cognitive impairment. Greater reduction in CT of the prefrontal areas involved in movement was detected in 7 pts with parkinsonism compared to the HCs. One L444P/V460V pt had an increased CT in 8/14 areas (prefrontal and temporo-occipital cortex). Alterations of CG were found in 6 areas of the frontal, posterior cingulate and temporal cortex. An increased CG in the posterior cingulate cortex areas, corresponding to the functional domains of attention, memory, motor control, sensory integration, and emotional response was detected in all GD1 pts. Moreover, 14/17 pts showed an increase of the frontal regions, corresponding to the functional domains of language, working memory, and movement. CG in a region of the secondary auditory cortex was reduced in GD1 pts greater than in HCs, whereas the CG in prefrontal and frontal regions, cingulate cortex, and inferior temporal gyrus were greater reduced in HCs compared to GD1 pts. Eight out of 16 pts with N370S/other genotype had CG values in 3/6 areas of the prefrontal and temporal cortex similar to those of HCs. Greater CG in 3/6 areas of the prefrontal, temporal and cingulate cortex was found in 4 pts with cognitive impairment. The CG in the frontal superior and cingulate cortex was found greater in 7 pts with parkinsonism signs than in HCs. All 9 symptomatic pts showed reduced CG in the temporo-occipital cortex compared to HCs.
CONCLUSIONS. The 3T MRI revealed that all GD1 pts had at least one significant alteration in CT and/or CG in 17/360 cortical regions, specifically in the prefrontal, posterior cingulate and temporo-occipital cortex. The reduction in CT and the increase in CG could be due to a process of brain atrophy. N370S mutation does not seem to be neuroprotective. Functional assessment of the brain areas could help to understand the mechanisms underlying the detected alterations. Additional instrumental and 3T MRI evaluations are planned to evaluate the evolution of cortical alterations and the development of clinical manifestations.
Disclosures
Giona:Sanofi: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.