Abstract
Background Myelodysplastic syndromes (MDS) are underreported and underdiagnosed, due to their rare occurrence and ambiguous presentation. Appropriate treatment selection, in light of an expanding therapeutic landscape, is highly dependent on an accurate diagnosis and risk stratification. To identify educational gaps limiting MDS risk stratification and individualized treatment selection, we surveyed hematology/oncology providers regarding clinical evidence on new and emerging therapies and challenges in care related to MDS treatment.
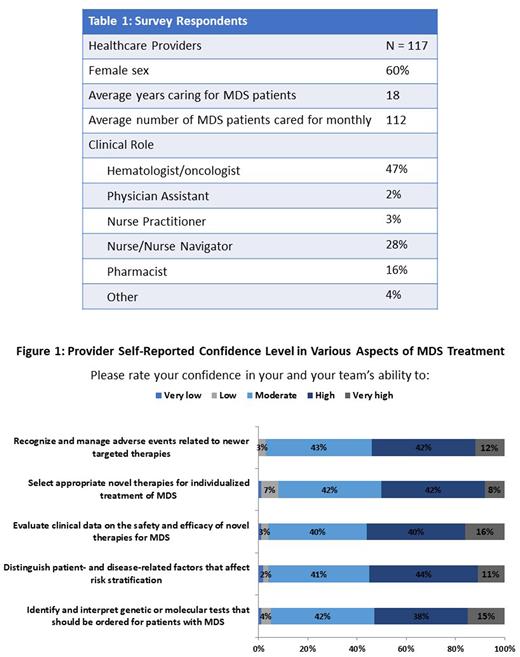
Methods During March 2022, a nationwide survey was conducted among 117 hematology/oncology healthcare providers (HCPs) caring for patients with MDS (Table 1). Survey questions were designed to identify knowledge and competence barriers in performing MDS risk assessment and treatment-decision making, as well as to provide a platform for HCPs to ask 'top-of-mind' questions in MDS care. Of the survey respondents, 47% were hematologists or oncologists, and providers reported an average monthly MDS caseload of 112 patients.
Results HCPs identified selecting optimal therapies for individual patients (35%) and obtaining an accurate diagnosis (22%) as the most challenging issue encountered in managing patients in MDS. In addition to clinical guidelines, HCPs considered treatment effectiveness (50%) and quality of life (50%) as the top two factors in treatment-decision making. When addressing barriers to adopting new therapies, HCPs reported being uncertain about who the "right" patients for new therapies are (35%) and having difficulty differentiating the roles of new therapies in the treatment armamentarium (20%). When asking HCPs to self-report their confidence levels in various aspects of MDS treatment, HCPs reported high confidence in their ability to identify and interpret genetic or molecular tests that should be ordered for MDS patients (53%), evaluate clinical data on the safety and efficacy of novel therapies for MDS (56%), and recognize and manage adverse events related to newer targeted therapies (54%) (Figure 1). 45% of HCPs reported moderate to low confidence in their ability to distinguish patient- and disease-related factors that affect risk stratification, and of this provider population, 40% stated that interpreting the correlation between cytogenetic abnormality and prognostic value is the biggest challenge impeding high confidence. Furthermore, of the 50% of HCPs that reported moderate to low confidence in selecting appropriate therapies for individualized MDS treatment, 47% stated their reported confidence was due to the challenge of keeping up with the clinical safety and efficacy therapy for new and emerging therapies. HCPs postulated key questions to MDS experts, including insight on the value of molecular tests in selecting novel therapies, how to individualize patient treatment plans, understanding the burden of transfusion-dependent anemia, timing for stem cell transplantation, and how to balance cost management and quality of life.
Conclusions HCPs disclosed key educational barriers that limit their ability to accurately diagnosis, risk stratify, develop individualized treatment plans, and manage treatment care for patients with MDS. Of note, HCPs revealed having difficulty differentiating the roles of the rapidly evolving therapies for MDS and identifying the patients that would benefit from them, which is exacerbated by limited confidence in incorporating cytogenetic findings into MDS patient risk assessment. To optimize MDS care, educational programming to inform HCPs on how to diagnose and risk stratify patients, as well as to report data on new and emerging therapies, is critical. These findings can inform future educational initiatives to close persistent gaps in quality MDS care.
Study Sponsor Statement The study reported in this abstract was funded by an independent educational grant from Gilead Sciences, Inc. The grantor had no role in the study design, execution, analysis, or reporting.
Disclosures
DeZern:Gilead: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Syntrix Pharmaceuticals: Research Funding; Bristol Myers Squibb: Consultancy, Honoraria; CTI BioPharma: Consultancy, Honoraria; GERON: Other: DSMB. Mesa:Genotech: Research Funding; Blueprint: Consultancy; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; AbbVie: Research Funding; CTI: Research Funding; AOP: Consultancy; Incyte: Consultancy, Research Funding; Geron: Consultancy; Samus: Consultancy, Research Funding; Promedior: Research Funding; LaJolla Pharmaceutical: Consultancy; Celgene: Research Funding; Bristol Myers Squibb: Consultancy; Sierra Oncology: Consultancy, Research Funding; Novartis: Consultancy; Roche: Consultancy; Gilead: Research Funding; Imago: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.