Abstract
BACKGROUND
Since the early 2000s, the standard treatment for CML-CP has been tyrosine kinase inhibitors (TKIs). Approximately 20% of CML patients fail to respond to first- and second-generation TKIs due to intolerance or treatment resistance, with limited treatment options being available for later-line settings. Asciminib received FDA approval in October 2021 for the treatment of adult patients with Philadelphia chromosome-positive (Ph+) CML-CP who were previously treated with two or more TKIs, and in patients with Ph+ CML-CP with the T315I mutation. Considering recent approval, no real-world data on the use of asciminib in clinical practice are available.
OBJECTIVE
This study aims to describe patient demographic and clinical characteristics, and initial treatment patterns among patients with CML-CP treated with asciminib in the real-world.
METHODS
This was a retrospective cohort study using IQVIA open-source pharmacy and medical claims data. Eligible patients had ≥ 1 pharmacy claim for asciminib between 01 November 2021 - 30 April 2022 (first claim date = index date), were at least 18 years of age on the index date and had at least 6 months of continuous pre-index data available. Demographic characteristics were assessed on the index date. Among a subgroup of patients with available medical claims baseline clinical characteristics were assessed during the fixed 6-month pre-index period, not including the index date. A claims-based proxy, defined as having prior therapy with ponatinib and a Current Procedural Terminology (CPT) code indicating mutation testing prior to or within 1-month of ponatinib initiation, was used to identify T315I mutation status.
RESULTS
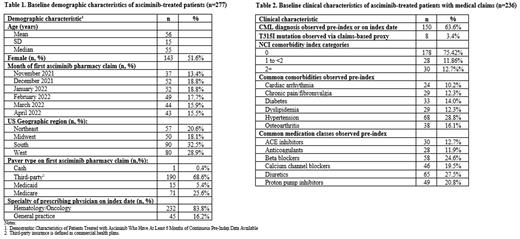
A total of 320 patients with asciminib pharmacy claims were identified; 277 (86.6%) had at least 6 months of pre-index data available. The mean (standard deviation, SD) age was 56 (15) years, 30.0% were ≥65 years old, 51.6% were female, and 32.5% were from the southern region of the US. Asciminib was mostly prescribed by hematologists and/or oncologists (83.8%). Most patients initiated therapy with a daily dose of 80 mg (86.1%) or 400 mg (8.9%) per the label-recommended dosing for patients in 3L+ and for patients with the T315I mutation, respectively. Overall, 67.2% of patients had at least one claim for another TKI during the 6 months prior to asciminib initiation, with the most commonly observed TKIs being ponatinib (23.1%), dasatinib (20.6%) and bosutinib (16.6%). The median time from discontinuation of the previous TKI and initiation of asciminib was 8 days. Evidence of non-TKI CML therapy (i.e., interferon, chemotherapy, or allogenic stem cell transplant) in the 6 months prior to asciminib initiation was observed in 11.9% of patients.
A total of 236 (73.6%) asciminib-treated patients had data for medical claims available. Among these, 63.6% had a diagnosis of CML on a medical claim observed during the 6-months prior to asciminib initiation (including index date) and 3.4% had the T315I mutation, based on a proxy measure, likely underestimating the true prevalence of this mutation. During the 6 months prior to asciminib initiation common comorbidities observed include hypertension (28.8%), osteoarthritis (16.1%) and diabetes (14.0%). Common medication classes observed during the 6 months prior to asciminib initiation include diuretics (27.5%), beta blockers (24.6%) and proton pump inhibitors (20.8%).
CONCLUSION
To our knowledge, this is the first study that describes real-world demographic and clinical characteristics of patients who initiated asciminib therapy immediately after launch in October 2021. Results show steady utilization of asciminib in clinical practice in patients with great unmet need as observed by the high comorbidity burden and high rates of medication use prior to initiation of asciminib. Results from this analysis are limited by the timeframe after launch and may not be representative of the broader asciminib-eligible CML population as treatment patterns evolve over time. Additional research with more mature data is needed to understand the true usage of asciminib in clinical practice and to assess outcomes in these patients.
Disclosures
Mukherjee:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant; Celgene/Acceleron: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Eusa Pharma: Consultancy, Other: Advisor or review panel participant; Teaching and Speaking; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisor or review panel participant, Research Funding; Aplastic Anemia and MDS International Foundation: Honoraria; McGraw Hill Hematology Oncology Board Review: Honoraria, Other: Advisor or review panel participant; Partnership for Health Analytic Research, LLC: Honoraria; BioPharm: Consultancy; Jazz Pharmaceuticals: Other: Principal investigator for Investigator Initiated Trials (the Institution gets the funding), Research Funding. Chen:Novartis: Consultancy; Takeda: Consultancy; GSK: Consultancy; Regeneron: Consultancy; Bristol Myers Squibb: Consultancy; Amgen: Consultancy; Pfizer: Consultancy. Gorritz:Novartis: Consultancy; Otsuka: Consultancy; Takeda: Consultancy; Eli Lilly: Consultancy. Sadek:Novartis: Current Employment, Current equity holder in publicly-traded company. Iorga:Novartis: Current Employment, Current equity holder in publicly-traded company. Zhou:GSK: Consultancy; Seqirus: Consultancy; Amgen: Consultancy; Novartis: Consultancy; Otsuka: Consultancy; Bristol Myers Squibb: Consultancy; Alexion: Consultancy; Galderma: Consultancy; Pfizer: Consultancy; Regeneron: Consultancy; Novo Nordisk: Consultancy. He:Horizon: Consultancy; Viiv: Consultancy; Ironshore: Consultancy; Protagonist: Consultancy; Novartis: Consultancy; Bayer: Consultancy; Daiichi Sankyo: Consultancy; GSK: Consultancy; Alexion: Consultancy; Blueprint: Consultancy; Ipsen: Consultancy. Maegawa:Novartis: Current Employment, Current equity holder in publicly-traded company. Damon:Novartis: Current Employment, Current equity holder in publicly-traded company. Ferreira:Novartis: Current Employment. Jadhav:Novartis: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.