Abstract
Introduction: Matched sibling or matched unrelated donor (MUD) availability remains a clinical challenge for hematopoietic cell transplant (HCT) recipients. Though HCTs with a mismatched unrelated donor (MMUD) may be an option, graft-versus-host disease (GVHD)-related mortality risk is elevated with MMUD HCTs. Abatacept (ABA), a selective T-cell costimulation modulator, when administered with calcineurin inhibitor/methotrexate (CNI/MTX), improved survival outcomes in 7/8 MMUD and 8/8 MUD recipients (Watkins B, et al. J Clin Oncol 2021) compared with CNI/MTX prophylaxis alone and was approved in the United States for acute GVHD prophylaxis (US FDA 2021). Alternative prophylactics, such as antithymocyte globulin (ATG) + CNI/MTX or post-transplant cyclophosphamide (PT-Cy) + tacrolimus (Tac) + mycophenolate mofetil (MMF) also show promise for improving post-HCT survival outcomes (Walker I, et al. Lancet Haematol 2020; Shaw B, et al. J Clin Oncol 2021; Luznik L, et al. J Clin Oncol 2022). This real-world analysis compared survival outcomes in 7/8 MMUD and 8/8 MUD recipients treated with ABA + CNI/MTX versus ATG + CNI/MTX and versus PT-Cy + Tac + MMF from the Center for International Blood and Marrow Transplant Research (CIBMTR) database of all allogeneic HCTs performed in the United States.
Methods: This retrospective cohort study included CIBMTR database allogenic HCT recipients treated with ABA + CNI/MTX, ATG + CNI/MTX, and PT-Cy + Tac + MMF (PT-Cy). Recipients were aged ≥ 6 years; weighed ≥ 20 kg; had a Karnofsky/Lansky performance score ≥ 80%; were diagnosed with leukemia, lymphoma, or myelodysplastic syndrome; and underwent first allogeneic HCT from a 7/8 MMUD or 8/8 MUD in the United States between 2011 and 2018. Overall survival (OS; time between HCT date and documented date of death) and relapse-free survival (RFS; time between HCT date and date of death or relapse, whichever occurred first) were evaluated over 1 year post-HCT. A weighted log-rank test was used to compare outcomes using inverse probability of treatment weighting (IPTW), based on propensity score calculation to control for treatment bias. Marginal hazard ratios (HRs) and 95% CIs were estimated by a weighted Cox proportional hazards model with treatment as a covariate.
Results: The 7/8 MMUD cohort included 378 recipients: 54 ABA + CNI/MTX, 162 ATG + CNI/MTX, and 162 PT-Cy. The 8/8 MUD cohort included 781 recipients: 71 ABA + CNI/MTX, 355 ATG + CNI/MTX, and 355 PT-Cy.
For 7/8 MMUD recipients, OS (95% CI) was significantly higher with ABA + CNI/MTX (87% [67-95%]) versus ATG + CNI/MTX (58% [49-66%]); P = 0.0026; HR (95% CI): 0.24 (0.10-0.55). The OS comparison between ABA + CNI/MTX versus PT-Cy was 86% (66-94%) and 79% (70-85%), respectively; P = 0.3376; HR (95% CI): 0.61 (0.25-1.51).
In 8/8 MUD recipients, OS (95% CI) was significantly higher with ABA + CNI/MTX versus ATG + CNI/MTX (86% [72-93%] versus 70% [65-75%], respectively); P = 0.0209; HR (95% CI): 0.43 (0.21-0.88). The OS comparison for ABA + CNI/MTX versus PT-Cy was 84% (66-93%) versus 78% (73-82%), respectively; P = 0.3616; HR (95% CI): 0.69 (0.30-1.54).
In 7/8 MMUD recipients, the ABA + CNI/MTX subgroup had significantly higher RFS versus ATG + CNI/MTX (81% [60-92%] versus 48% [39-56%], respectively); P = 0.0015; HR (95% CI): 0.27 (0.13-0.55). The RFS comparison between ABA + CNI/MTX versus PT-Cy was 75% (56-87%) versus 62% (52-70%), respectively; P = 0.1636; HR (95% CI): 0.60 (0.26-1.39).
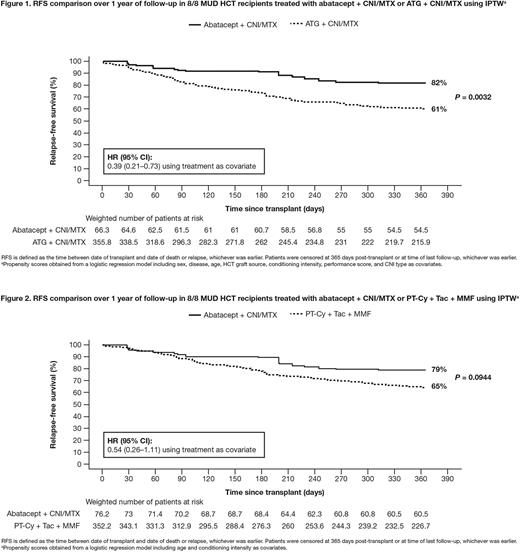
For 8/8 MUD recipients, Figure 1 shows ABA + CNI/MTX had significantly higher RFS versus ATG + CNI/MTX (82% [69-90%] versus 61% [55-66%], respectively); P = 0.0032; HR (95% CI): 0.39 (0.21-0.73). Figure 2 shows the RFS comparison between ABA + CNI/MTX and PT-Cy (79% [61-90%] versus 65% [60-70%], respectively); P = 0.0944; HR (95% CI): 0.54 (0.26-1.11).
Conclusions: ABA + CNI/MTX significantly improved OS and RFS over 1-year post-HCT compared with ATG + CNI/MTX prophylaxis. There was a trend toward improved OS and RFS 1-year post-HCT on ABA + CNI/MTX compared with PT-Cy, although this did not reach statistical significance. OS and RFS in 7/8 MMUD recipients receiving ABA + CNI/MTX was similar to those in 8/8 MUD recipients, suggesting that this treatment improved the risk/benefit ratio for MMUD HCTs.
Study support: This study was sponsored by Bristol Myers Squibb. Medical writing: Efrah Yousuf and Ryan Miller (Caudex), funded by Bristol Myers Squibb. We acknowledge Brian Gavin, PhD, for his contributions.
Disclosures
Kean:Bristol Myers Squibb: Patents & Royalties: clinical trial, Research Funding; HiFiBio: Consultancy; Mammoth Biosciences: Current equity holder in private company, Current holder of stock options in a privately-held company; Bluebird Bio: Research Funding; Novartis: Research Funding; EMD-Serono: Research Funding; Magenta: Research Funding; Vertex: Consultancy. Burns:Astellas Pharma Inc.: Research Funding; Bristol Myers Squibb: Research Funding; Gamida Cell: Research Funding. Kou:BeiGene, USA, Inc.: Current Employment; Bristol Myers Squibb: Ended employment in the past 24 months. Bond:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Kapikian:Cytel: Current Employment, Other: On loan to Bristol Myers Squibb. Lozenski:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Tang:Bristol Myers Squibb: Research Funding; Gamida-Cell Ltd: Research Funding; Kite Pharma: Other: Spouse is currently employed at Kite. Zhang:Gamida Cell: Research Funding; Bristol Myers Squibb: Research Funding; Astellas Pharma Inc: Research Funding. Hemmer:Kite Pharma: Current Employment; Pfizer: Honoraria. Connolly:Bristol Myers Squibb: Current Employment, Current equity holder in private company. Polinsky:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Gomez:Bristol Myers Squibb: Current Employment. Pasquini:Novartis: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Janssen: Research Funding; Kite: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.