Abstract

Aim: Vaccine induced thrombosis thrombocytopenia (VITT) syndrome is a rare complication of ChAdOx1 vaccination for COVID19. VITT shares similarities with heparin induced thrombocytopenia (HIT). In both VITT and HIT, patients develop antibodies against platelet factor 4 (PF4) which promote platelet activation and thrombosis. Involvement of the endothelium in the pathogenic process has been described for HIT. Binding of HIT antibodies to PF4/glycosaminoglycan complexes on the surface of endothelial cells leads to their activation and increased procoagulant activity (Madeeva, J Thromb Haemost, 2016). However, the contribution of the endothelium to VITT has not been described. Here, we evaluate the effect of VITT plasma or serum on endothelial cell activation and thromboinflammation using an endothelialized biochip.
Method: Serum and plasma samples were received from patients fulfilling clinical criteria of VITT including venous and/or arterial thrombosis, thrombocytopenia <150 x 10^3/uL, D-dimer >5-fold of upper limit of normal within 4-42 days after 1st dose of ChAdOx1 vaccine (termed "Clinical VITT") and from healthy, age and sex matched, ChAdOx1 vaccinated individuals within the same timeframe from vaccination (termed "Vaccine controls"). PF4 ELISA and VITT functional assays were performed on all samples (Lee, Blood Adv, 2022). Human umbilical vein endothelial cells (HUVECs) were treated with heat inactivated serum or plasma in the presence of peptide GPRP. Polydimethylsiloxane microfluidic straight channels were seeded with HUVECs (Endochip) and treated with VITT or control serum and compared with TNF alpha treatment. The endothelial surface expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), P-selectin, tissue factor (TF) and von Willebrand factor (vWF) were measured by immunostaining in the presence or absence of native platelet factor 4 (PF4). In some experiments HUVECs were treated with ChAdOx1 vaccine or vehicle for 48-120h. Thromboinflammation was measured by fluorescence area of antibody-stained platelets, neutrophils and fibrin on the Endochip after perfusion of recalcified blood from HIT antibody-reactive healthy volunteers (Morel-Kopp, J Thromb Haemost, 2016) at a shear rate of 100s-1.
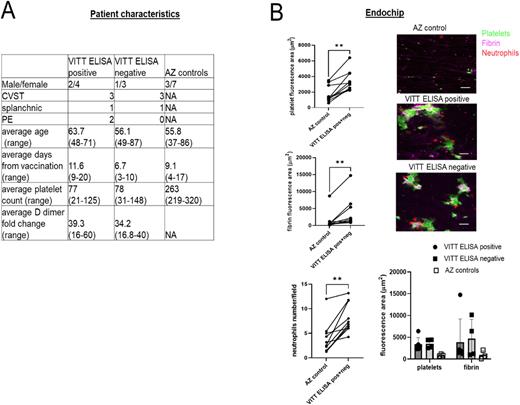
Results: Samples from 10 patients presenting with clinically confirmed VITT were paired with samples from 10 vaccinated individuals. Clinical VITT samples were separated into negative and positive for both PF4 ELISA and VITT functional assay (defined as VITT ELISA positive and VITT ELISA negative, Panel A). Endothelial cell surface expression of P-selectin was increased after 12 h incubation with 25% v/v VITT serum or plasma compared with samples from AZ vaccinated non-VITT individuals (median integrated intensity/cell 8065 vs 3515 a.u, p=0.03). The expression of TF was significantly increased by addition of PF4 to the VITT plasma (median integrated intensity/cell 42038 vs 14881 a.u., p=0.01 by Mann Whitney test). ChAdOx1 treatment of HUVECs at 1:1000 dilution induced the expression of spike protein but did not significantly enhance thromboinflammation compared with media, as assessed by the perfusion of whole blood through the Endochip. Treatment of the Endochip with VITT serum, without the addition of PF4, significantly increased the adhesion of platelets compared with control serum (median 2.2-fold, p=0.002), neutrophils (2.5-fold, p=0.003) and fibrin (5.4-fold, p=0.002 by Wilcoxon paired samples test (Panel B). There was no difference in the thromboinflammatory response between ELISA negative and positive group (Panel B).
Conclusion: Endothelial inflammation contributes to the prothrombotic tendency in VITT in an in vitro model of thromboinflammation. VITT serum induces the endothelial expression of P-selectin and TF. Endothelial inflammation may explain thrombotic events in cerebral or splanchnic veins of vaccinated individuals without detectable anti-PF4 antibodies by ELISA or platelet-based VITT functional assays. Endothelialised biochips have diagnostic potential for detecting immune prothrombotic tendencies.
Disclosures
Tran:Pfizer, Takeda: Speakers Bureau; AstraZeneca, CSL Behrig: Honoraria; Sanofi: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.