Abstract
Background: Endothelial toxicity (by toxins, viral and bacterial sepsis) and associated inflammation can lead to acute and chronic vasculopathy leading to cardiac compromise. CD39 helps to maintain the endogenous antithrombotic profile of the endothelium. CD39 is an endothelial cell membrane-anchored enzyme that hydrolyses ATP and ADP to AMP. Subsequently, another enzyme CD73 further hydrolyses AMP to adenosine. The consequence of this important pathway is that pro-inflammatory (ATP) and pro-thrombotic (platelet aggregation amplifier, ADP) are converted to adenosine, which is anti-inflammatory, vasodilatory, and anti-thrombotic. CD39 enzyme activity drives this pathway.
We have published before that CD39 is anti-thrombotic/anti-inflammatory (N Engl J Med. 2012;367(24):2322-2333; J Clin Invest. 2004;113(10):1440-1446) and also protects from ischaemia-reperfusion injury in heart and kidney (Eur Heart J. 2018;39(2):111-116; Am J Transplant. 2010;10(12):2586-2595)
As a human disease correlate, CD39 activity and adenosine signalling is disrupted in human pulmonary hypertension (J Am Heart Assoc. 2020;9:e017404). Moreover, CD39-null mice are predisposed towards the development of experimental pulmonary hypertension after hypoxic injury (Am J Physiol Heart Circ Physiol 311: H286-H298, 2016).
In this work, we demonstrate that the administration of a single dose of an endothelial cell toxin (monocrotaline pyrrole) leads to the development of vasculopathy and pulmonary hypertension as characterised by cardiac echo and right ventricular pressure studies.
Single administration of endothelial (Vascular cell adhesion molecule, VCAM-1)-targeted soluble CD39 (Anti-VCAM-CD39) ameliorates pulmonary hypertension.
Hypothesis: Targeted delivery of CD39 to the pulmonary endothelium will relieve pulmonary hypertension.
Methods: 'Anti-VCAM-CD39' is novel therapeutic that contains a scFv recognising VCAM-1 fused to human soluble CD39. It is expressed as a single polypeptide from insect or mammalian cells and affinity purified using a FLAG-affinity tag. Prior work has demonstrated that Anti-VCAM-CD39 administration intravenously leads to dose-dependent prolongation of murine tail bleeding time only at doses above 1mg/kg.
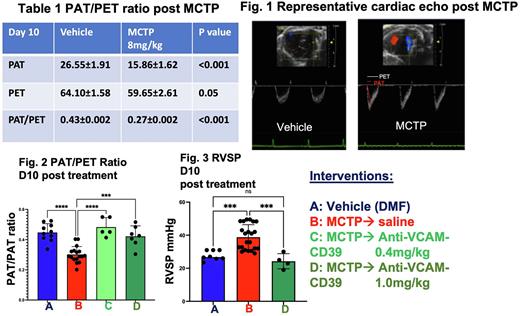
We have developed a murine model of pulmonary hypertension by a single administration of monocrotaline pyrrole (MCTP, 8mg/kg). Development of pulmonary hypertension was verified by cardiac echo showing abnormal (decreased) pulmonary acceleration time (PAT): pulmonary ejection (PET) ratio (an accepted parameter of pulmonary hypertension and increased heart weight ratio (heart weight/body weight x100). Pulmonary hypertension was also confirmed by right ventricular systolic pressure (RVSP) measurement.
Cardiac echo was done with the Vevo2100 small animal high-frequency ultrasound machine and the 55Mhz MS550s transducer.
RVSP was quantified in anaesthetised mice using a Millar pressure catheter (1.4Fr) inserted into the RV, via the right external jugular vein.
Results:Development of pulmonary hypertension with single dose of MCTP 8mg/kg shows features of: 1. Increased endothelial VCAM-1 expression (mRNA expression, not shown)
2. Decreased PAT/PET ratios consistent with pulmonary hypertension (Table 1) with typical cardiac echo appearance (Fig. 1)
3. Increased cardiac weight:body weight ratio at D21 (vehicle 0.45±0.05; MCTP 0.6±0.1, p<0.05)
4. Leads to fibrin deposition (Carstairs stain, not shown).
Anti-VCAM-CD39 ameliorates pulmonary hypertension, quantitated with cardiac echo and RVSP: 1.Single administration of Anti-VCAM-CD39 at doses of 0.4mg/kg and 1.0mg/kg at 72 h post MCTP improved (increased) PAT/PET ratio at D10 compared with saline control (Fig. 2).
2. Single administration of Anti-VCAM-CD39 at 1.0mg/kg reduced right ventricular systolic pressure (RVSP mmHg) at D10 consistent with amelioration of pulmonary hypertension. (Fig. 3). Effect of lower dose of Anti-VCAM-CD39 at 0.4mg/kg is awaited.
Conclusion: 1. MCTP administration cause endothelial activation, VCAM-1 expression and vascular remodelling leading to a model of pulmonary hypertension with right ventricular pressure overload.
2. Single administration of Anti-VCAM-CD39 at doses 0.4mg/kg and 1.0mg/kg (below the threshold prolonging tail-bleeding time) leads to amelioration of pulmonary hypertension suggesting a novel new treatment for this condition.
Disclosures
Robson:Purinomia Biotech Inc: Other: Scientific Founder; eGenesis: Consultancy; Abbvie: Consultancy; SynLogic Inc: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.