Abstract
Background: The curative potential of allogeneic transplant (alloHCT) in patients with acute lymphoblastic leukemia (ALL) is hampered by relapse, the major cause of treatment failure. Risk factors for relapse include measurable residual disease (MRD) before or after alloHCT, transplantation in second complete remission (CR) or beyond, and reduced intensity conditioning (RIC) regimens. In ALL patients, relapse rates range from 30% to 50%, with most relapses occurring within the first year after alloHCT. After relapse, options for disease control are limited, and consequently overall survival (OS) is poor. Post-alloHCT maintenance may reduce MRD, provide time for a graft-versus-leukemia effect to develop, and ultimately decrease relapse rates and prolong progression-free-survival (PFS) and overall survival (OS).
Inotuzumab ozogamicin (INO) is an anti-CD22 monoclonal antibody bound to calicheamicin and approved to treat relapsed ALL. With the hypothesis that low-dose INO would be safe and reduce relapse rates, we conducted a phase I study to define INO dose in this setting. Herein we present the results of this multicenter study.
Methods: Eligibility included patients aged 16-75 who underwent alloHCT for CD22+ALL and were in CR after transplant, had a high risk for relapse after alloHCT (MRD before or after alloHCT, transplantation,in second CR or beyond, RIC regimen, lymphoid blast crisis of chronic myeloid leukemia, or Ph-like ALL), had adequate graft and organ function after transplant (platelets > 50 ANC > 1000), did not have active grade III/IV aGVHD or any active liver GVHD, and had no history of hepatic VOD. Patients were consented after transplant. The primary objective was to define a post-alloHCT maximum tolerated dose (MTD) of INO. Secondary objectives included describing the safety profile of INO, determining the rate of hepatic veno-occlusive disease (VOD) in this setting, evaluating non-relapse mortality (NRM), PFS and OS at 1-year post-alloHCT, and determining if INO at these doses is effective at eradicating MRD.
Pre-treatment tests included a bone marrow biopsy and aspirate to assess disease state and MRD. The trial design was a 3+3 model. The study treatment consisted of 28-day cycles (initially planned for 4 cycles, increased up to 12 cycles) of INO starting at dose level 0 (0.3mg/m2; n= 4), then 0.4 mg/m2 (n= 3), 0.5 mg/m2 (n= 6) and 0.6 mg/m2 (n= 5) given on cycle day 1. The first cycle was initiated between day 40 and 100 post-alloHCT. Pre-defined dose limiting toxicities (DLT) included VOD, prolonged cytopenia (more than 28 days), severe toxicities ascribed to INO, and death. If there were no DLTs experienced in first two cycles of a cohort, subsequent patients were enrolled in the next highest dose level.
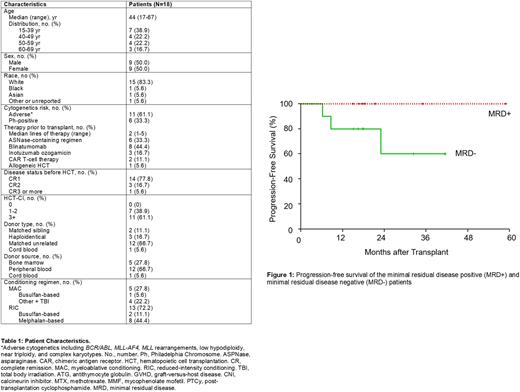
Results: We have enrolled 19 patients (18 treated, Table 1) with a median follow up of 18.1 months and a median number of 3 doses (range, 1-9). Only treated patients are included in analyses. The median time to first INO dose was 84 days (range, 47-100) after alloHCT. There was one DLT (prolonged thrombocytopenia) and the study reached the final pre-defined dose of 0.6mg/m2. No VOD was observed. The most common toxicity was thrombocytopenia (42.1% of patients; 31.6% experienced grade 3 or 4 thrombocytopenia), neutropenia (31.6% of patients, 15.8% experienced grade 3 or 4 neutropenia), and nausea/vomiting (42% of patients, all grade 1 or 2). 68% of patients received reduced intensity or non-myeloablative conditioning.
OS at 1-year post-alloHCT was 94.4% and PFS was 88.9%. One-year NRM was 5.5%. One patient died of aGVHD on 0.6mg/m2 and one patient relapsed on 0.5mg/m2 at 7 months post-alloHCT. Another patient relapsed from Ph-like ALL 23 months post-alloHCT. Seven of the patients enrolled either had MRD before (n = 4) or after alloHCT (n = 3) as measured by flow cytometry, PCR, or NGS. For patients with MRD before or after alloHCT, 1-year OS and PFS are 100% (Figure 1). The 1-year PFS and OS of recipients of RIC transplants was 83.3% and 91.7%.
Conclusions: We have determined that INO at doses of 0.3, 0.4, 0.5 and 0.6mg/m2 was well tolerated and that 0.6 mg/m2 is the MTD. Thrombocytopenia may be the DLT. The low observed relapse rate and favorable safety profile justify investigating low-dose INO as maintenance therapy after alloHCT in a phase II trial.
Disclosures
Metheny:Gamida: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau. Caimi:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees. Malek:Sanofi: Consultancy; AdaptiveBio: Consultancy, Honoraria; Amgen: Speakers Bureau; BMS: Speakers Bureau; Takeda: Consultancy, Speakers Bureau; janssen: Consultancy; GSK: Speakers Bureau; Karyopharm: Consultancy, Speakers Bureau. Gerds:Accurate Pharmaceuticals: Research Funding; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte Corporation: Research Funding; Kratos Pharmaceuticals: Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago BioSciences: Research Funding; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Hamilton:Syndax: Membership on an entity's Board of Directors or advisory committees; Kadmon: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Nkarta: Membership on an entity's Board of Directors or advisory committees; Equilium: Membership on an entity's Board of Directors or advisory committees. Giralt:Spectrum Pharma: Consultancy; Kite: Consultancy; Omeros: Research Funding; Novartis: Consultancy; Takeda: Consultancy, Research Funding; Miltenyi: Research Funding; Jazz Pharmaceutical: Consultancy; Janssen: Consultancy; Johnson & Johnson: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Actinuum: Consultancy, Research Funding. Perales:Abbvie: Honoraria; Sellas Life Sciences: Consultancy; DSMB: Other; Miltenyi Biotec: Consultancy, Honoraria; Bellicum: Honoraria; Takeda: Honoraria; Karyopharm: Honoraria; MorphoSys: Consultancy, Honoraria; Celgene: Honoraria; Astellas: Honoraria; Bristol-Mysers Squibb: Honoraria; Medigene: Consultancy; Novartis: Honoraria; VectivBio AG: Honoraria; Nektar Therapeutics: Consultancy, Honoraria; Omeros: Consultancy; Vor Biopharma: Honoraria; Kite, a Gilead Company: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Servier: Consultancy; Orca Bio: Consultancy; Cidara Therapeutics: Consultancy; Merck: Consultancy. de Lima:Pfizer: Consultancy, Research Funding; Incyte: Consultancy; Amgen: Consultancy; Celgene: Consultancy, Research Funding.
OffLabel Disclosure:
Post-Transplant Low-dose Inotuzumab Ozogamicin to Prevent Relapse of Acute Lymphoblastic Leukemia
Author notes
Asterisk with author names denotes non-ASH members.