Abstract
Background: Patients (pts) with higher-risk MDS need tolerable treatment options with durable clinical benefits. Sabatolimab (MBG453) is a novel immunotherapy targeting T-cell immunoglobulin domain and mucin domain-3 (TIM-3), an immuno-myeloid regulator expressed on both immune cells and leukemic stem cells. Sabatolimab+HMAs delivered durable responses in a Phase (Ph) Ib study in pts with high-risk/very high risk (HR/vHR)-MDS (Brunner AM, ASH 2021). Here, we report the primary results from the ongoing STIMULUS-MDS1 (NCT03946670), a randomized, double-blind, placebo-controlled, Ph II study of sabatolimab+HMA in pts with intermediate risk (IR; and ≥5% bone marrow [BM] blasts), HR, or vHR-MDS who were ineligible for intensive chemotherapy or hematopoietic stem cell transplant at screening.
Methods: Treatment-naive pts aged ≥18 years with IR/HR/vHR-MDS as defined by the Revised International Prognostic Scoring System (IPSS-R) were eligible. Pts were randomized 1:1 to sabatolimab+HMA or placebo+HMA and stratified by risk category (per investigator assessment) and type of HMA (azacitidine [AZA] or decitabine [DEC]; per investigator discretion). Intravenous (IV) sabatolimab (400 mg) or placebo were administered on Day (D)8 and D22 every 2 weeks, AZA (75 mg/m2 IV or subcutaneous) on D1-7 or D1-5 + D8-9, or DEC (20 mg/m2 IV) on D1-5. The 2 primary endpoints were complete remission (CR, per modified International Working Group-MDS criteria) rate and progression-free survival (PFS: time from randomization to relapse from CR, progression [including acute myeloid leukemia], or death). Secondary endpoints included response rates, overall survival (OS), duration of response, and safety.
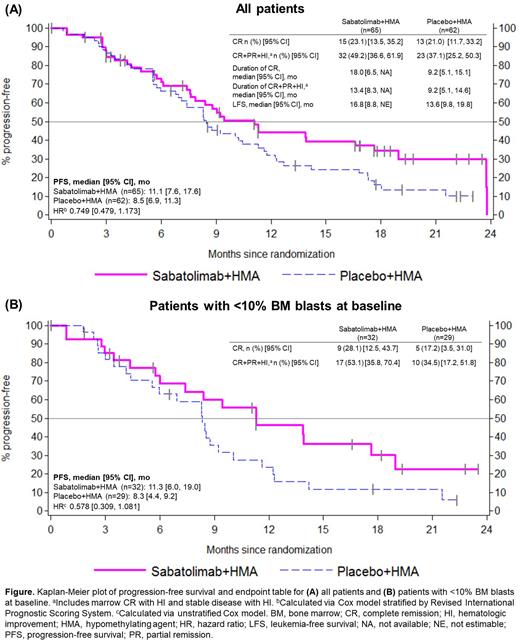
Results: A total of 127 pts were randomized (sabatolimab+HMA, N=65; placebo+HMA, N=62) between 29 JUL 2019 and 10 AUG 2020; 89% received AZA and 11% DEC. Reported results are from the primary analysis, when the study was unblinded (data cutoff 1 MAR 2022), unless noted otherwise. Median follow-up was 24 months (mo; randomization to cutoff). Baseline characteristics were similar between arms; median age was 73 years; 16.5%, 37.8%, and 45.7% of pts had IR, HR, and vHR-MDS, respectively. Median PFS was (sabatolimab+HMA vs placebo+HMA) 11.1 vs 8.5 mo (P=0.102, 1-sided; not significant [ns]; Figure A). Primary CR rate based on data up to 7 mo after the last pt's first visit was evaluated earlier by an independent data monitoring committee; CR rate was (sabatolimab+HMA vs placebo+HMA) 21.5% (14/65) vs 17.7% (11/62) (P=0.769, 1-sided; ns). Updated CR rate at primary analysis was (sabatolimab+HMA vs placebo+HMA) 23.1% vs 21.0%, CR+partial remission (PR)+hematologic improvement (HI; includes marrow CR with HI and stable disease with HI) was 49.2% vs 37.1%, median duration of CR was 18.0 vs 9.2 mo, median duration of CR+PR+HI was 13.4 vs 9.2 mo, and OS was 19.0 vs 18.0 mo (hazard ratio 0.905 [95% CI: 0.565, 1.450]). In an exploratory subgroup analysis of pts with <10% BM blasts at baseline (sabatolimab+HMA, n=32; placebo+HMA; n=29), CR was 28.1% vs 17.2%; CR+PR+HI was 53.1% vs 34.5%; and median PFS was 11.3 vs 8.3 mo (Figure B). Similar beneficial results were seen in IR/HR IPSS-R pts. The grade ≥3 adverse events (AEs) with >20% in either arm were (sabatolimab+HMA vs placebo+HMA) neutropenia (53.2% vs 63.5%), thrombocytopenia (37.1% vs 42.9%), febrile neutropenia (35.5% vs 23.8%), anemia (22.6% vs 42.9%), and leukopenia (22.6% vs 28.6%). The most common non-hematological all grade AEs were constipation (46.8% vs 38.1%) and diarrhea (43.5% vs 22.2%). One pt developed a serious potential immune-mediated AE (pneumonitis) on study treatment (sabatolimab+DEC; 6 doses of 400 mg of sabatolimab) and was treated with immunosuppressants including steroids. This AE was suspected as study treatment related and resulted in fatality.
Conclusions: Sabatolimab+HMA is associated with a favorable safety profile in pts with higher-risk MDS. Although improvements in CR and PFS were not statistically significant, when assessed with the better duration of response, the data may suggest a delayed-onset benefit in the sabatolimab arm, as well as a preferential effect in pts with lower disease burden. Additional exploratory and biomarker analyses will be reported in the meeting. The ongoing Ph III STIMULUS-MDS2 with a primary endpoint of OS (NCT04266301) has completed accrual and will definitively inform on the impact of sabatolimab in higher-risk MDS.
Disclosures
Zeidan:Novartis, Cardiff Oncology, Pfizer: Other: Travel Support; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie: Consultancy, Honoraria, Other: Advisory Board; Celgene/BMS, Novartis, AbbVie, Gilead, Kura, Loxo Oncology, Geron: Other: Clinical Trial Committee; Celgene/BMS, Novartis, Cardiff Oncology, AbbVie, Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Astex, Pfizer, Medimmune/AstraZeneca, ADC Therapeutics: Research Funding; Gilead, Kura, Loxo Oncology: Consultancy, Honoraria, Other: Clinical Trial Committee; Celgene/BMS, AbbVie, Pfizer, Boeringer-Ingelheim, Trovagene, Cardiff Oncology, Incyte, Takeda, Novartis, Aprea, Amgen, Otsuka: Consultancy, Honoraria, Research Funding; Astex, Medimmune, Astrazeneca, ADC Therapeutics: Research Funding; Jazz, Agios, Acceleron, Astellas, Daiichi Sankyo, Cardinal Health, Taiho, Seattle Genetics, Beyondspring, Gilead, Kura, Tyme, Janssen, Syndax, Geron, Ionis, Epizyme: Consultancy, Honoraria; Pfizer, Boehringer-Ingelheim, Trovagene, Incyte, Takeda, Amgen, Aprea, Gilead, Kura, Loxo Oncology, Otsuka, Jazz, Agios, Acceleron, Astellas, Daiichi-Sankyo, Cardinal Health, Taiho, Seattle Genetics, BeyondSpring, Ionis, Epizyme, Janssen, Syndax, Genentec: Consultancy, Honoraria, Other: Advisory Boards. Ando:Astella: Honoraria; Celgene: Honoraria; Novartis international: Honoraria; Kyowa Kirin: Research Funding; Takeda Pharmaceutical: Research Funding; CHUGAI PHARMACEUTICAL: Research Funding. Rauzy:Celgene/BMS, Novartis: Research Funding. Cairoli:Novartis, Celgene: Speakers Bureau; Celgene, AbbVie, Daiichi Sankyo, Novartis: Consultancy; Gilead Sciences: Other: Travel, Accommodations. Hou:AbbVie, Celgene, Kirin: Research Funding; AbbVie, Astellas, AstraZeneca, BMS, Chugai, CSL Behring, Daiichi Sankyo, IQVIA, Johnson & Johnson, Kirin, Lotus, Merck Sharp & Dohme, Novartis, Ono, Panco Healthcare Co, Pfizer, PharmaEssential, Roche, Synmosa, Takeda, TSH Biopharm, TTY Biopharm Company, : Consultancy, Honoraria, Other: Travel. Kwong:Novartis, Merck, Bristol Meyer Squibb: Research Funding; Amgen, Astellas, Bayer, Beigene, Bristol Meyer Squibb, Celgene, Janssen, Merck, Otsuka, Novartis, Roche, Takeda: Consultancy, Speakers Bureau. Meers:Alexion, AbbVie, BMS/Celgene, Janssen, Novartis, Takeda: Consultancy, Honoraria, Other: Advisory Board Member. Pullarkat:Amgen, Dova, and Novartis: Consultancy, Other: Advisory Board Member; AbbVie, Amgen, Genentech, Jazz Pharmaceuticals, Novartis, Pfizer, and Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Santini:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Otsuka: Honoraria, Membership on an entity's Board of Directors or advisory committees; Geron: Honoraria, Membership on an entity's Board of Directors or advisory committees; Menarini: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria; Novartis: Honoraria; Syros: Membership on an entity's Board of Directors or advisory committees; Srvier: Membership on an entity's Board of Directors or advisory committees. Malek:Novartis Pharma AG: Current Employment. Kiertsman:Novartis Pharmaceuticals Corporation: Current Employment. Lyu:Novartis, China: Current Employment. Marques Ramos:Novartis Pharma AG: Current Employment. Fenaux:Jazz: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene/BMS: Honoraria, Research Funding; Syros Pharmaceuticals: Honoraria. Miyazaki:Otsuka Pharmaceutical: Honoraria; Pfizer: Honoraria; Kyowa-Kirin: Honoraria; Bristol-Myers: Honoraria; Chugai: Honoraria; SyinBio: Honoraria; Dainippon-Sumitomo Pharma: Honoraria, Research Funding; Astellas: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Nippon Shinyaku: Honoraria; Takeda: Honoraria; Daiichi-Sankyo: Honoraria; Janssen Pharmaceutical: Honoraria; Celgene: Honoraria. Platzbecker:Jazz: Honoraria; Janssen: Honoraria; Silence Therapeutics: Honoraria; Novartis: Honoraria; Takeda: Honoraria; BMS/Celgene: Honoraria; Abbvie: Honoraria; Geron: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.