Abstract
Background Clonal hematopoiesis (CH), a precursor to myeloid malignancies, is extremely common. Formally defined CH subtypes, categorized by somatic mutations in genes associated with myeloid neoplasia (MN) at a variant allele fraction (VAF) ≥ 0.02, include clonal hematopoiesis of indeterminate potential (CHIP) where no cytopenia is present and clonal cytopenia of uncertain significance (CCUS). The rate of diagnosis of CHIP and CCUS is increasing due to widespread use of next generation sequencing (NGS) to evaluate unexplained cytopenias and "liquid biopsies" for patients with solid malignancies. Given the low rate of transformation to overt MN, defining the clinical and genetic factors that impact malignant evolution and developing a comprehensive prognostic model would aid clinical management of this growing patient population.
Methods We analyzed whole exome sequencing data from 193,743 healthy volunteer participants in the UK Biobank (UKB) who met study inclusion criteria. Of these, 11,337 (5.85%) had pathogenic variants in genes associated with myeloid malignancies and otherwise met criteria for CHIP or CCUS (CHIP/CCUS). We used genetic mutations, blood cell parameters and clinical outcomes in conditional probability based recursive partitioning analysis to identify a set of variables critical for classifying CHIP/CCUS cases into groups with distinct risk of MN. Statistical weights of identified variables were derived using multivariable Cox regression with sex, smoking history, and prior cancer history as confounders. The sum of the statistical weight of each prognostic variable defined a clonal hematopoiesis risk score (CHRS). We tested the CHRS model in hematology patient datasets, including a cohort of 646 CHIP/CCUS patients evaluated at Dana-Farber/Brigham and Women's Hospital (DFCI/BWH) and an independent cohort of 99 CCUS patients evaluated at the University of Pavia.
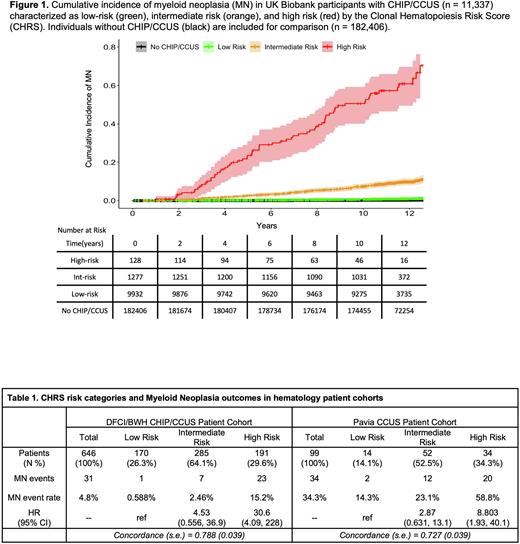
Results We classified 10,479 individuals in the UKB (92.4% of CHIP/CCUS cases) as CHIP and 858 (7.6%) individuals with unexplained cytopenias as CCUS. The majority of CHIP/CCUS cases had only one CH mutation (n = 10,352, 91.1%) and 6,235 cases (55.0%) involved a single DNMT3A mutation. Median follow-up was 11.5 years and a total of 246 incident MN events were observed. Recursive partitioning analysis distinguished groups of CHIP/CCUS cases with 10-year probabilities of incident MN ranging from 0.0078 - 0.65, highlighting marked variability of MN risk. Identified partitioning variables included genetic features (single DNMT3A mutation, high risk mutations, > 1 mutation, and VAF ≥ 0.2), patient age, CCUS vs CHIP, and high red blood cell indices (red cell distribution width and mean corpuscular volume). These variables were integrated to create a simple prognostic model for CH. The CHRS robustly characterizes the majority of CHIP/CCUS cases in UKB as low risk for incident MN (n = 9,932, 87.6%). Of the remaining cases, 11.3% (n =1,277) were intermediate risk and a minority of cases (n= 128, 1.13%) were high risk. A low rate of MN was observed in the 182,406 individuals without CHIP/CCUS, where the 5-year cumulative incidence of MN (± standard deviation) was 0.074 ± 0.00639% and the 10-year cumulative incidence was 0.210 ± 0.108%. Among CHRS risk groups, the respective 5- and 10- year cumulative incidences for MN in CHIP/CCUS were 0.234 ± 0.0487% and 0.674 ± 0.0834% in low risk, 2.59 ± 0.452% and 7.34 ± 0.759% in intermediate risk, and 23.6 ± 3.99% and 50.6 ± 4.89% in high risk (Figure 1). While the UKB is a population cohort, patients evaluated for CHIP/CCUS in hematology clinics may have an inherently higher risk of developing MN. We therefore tested the CHRS in two independent cohorts of CHIP/CCUS patients evaluated with clinical NGS in hematology clinics at tertiary referral centers. The overall MN event rate was 4.8% in the DFCI/BWH CHIP/CCUS cohort and 34.3% in the Pavia CCUS cohort, underscoring the enhanced risk of progression in CCUS compared to CHIP. In both patient cohorts, the majority of patients who progressed to MN were classified as high risk by the CHRS with model concordance (C-index) of > 0.7 (Table 1).
Conclusions The CHRS provides a simple and reproducible prognostic framework for CH, clearly stratifying CHIP/CCUS cases at the population and patient levels. Use of this model enables distinction of a high risk minority from the majority of individuals with CH who have minimal risk for progression to overt MN.
Disclosures
Neuberg:Madrigal Pharmaceuticals: Current equity holder in private company. Luskin:Abbvie: Research Funding; Pfizer: Honoraria; Novartis: Research Funding. Stone:Epizyme: Consultancy; Foghorn Therapeutics: Consultancy; Aprea: Consultancy; Elevate Bio: Consultancy; BMS: Consultancy; Apteva: Consultancy; BerGenBio: Consultancy; Astellas: Consultancy; Janssen: Consultancy; Novartis: Consultancy; Innate: Consultancy; Jazz: Consultancy; GSK: Consultancy; Gemoab: Consultancy; Kura Oncology: Consultancy; OncoNova: Consultancy; Syndax: Consultancy; Boston Pharmaceuticals: Consultancy; Actinium: Consultancy; Arog: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Syntrix: Consultancy; Syros: Consultancy; Takeda: Consultancy. DeAngelo:Abbvie: Research Funding; Agios: Consultancy; Blueprint Medicines Corporation: Consultancy; Incyte: Consultancy; Forty-Seven: Consultancy; Autolus: Consultancy; Amgen: Consultancy; Glycomimetics: Research Funding; Shire: Consultancy; Takeda: Consultancy; Novartis: Consultancy, Research Funding; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy. Soiffer:Juno: Membership on an entity's Board of Directors or advisory committees; VOR Biopharma: Consultancy; Jazz: Consultancy; Rheos Therapeutics: Consultancy; Gilead: Consultancy; Be The Match/National Marrow Donor Program: Membership on an entity's Board of Directors or advisory committees; Kiadis: Membership on an entity's Board of Directors or advisory committees; Alexion: Consultancy. Bick:TenSixteen Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Jaiswal:Novartis: Consultancy, Honoraria; Roche-Genentech: Consultancy, Honoraria; AVRO Bio: Consultancy, Honoraria; GSK: Consultancy, Honoraria; Bitterroot Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; TenSixteen Bio: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees. Natarajan:Amgen: Research Funding; Apple: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Boston Scientific: Research Funding; Novartis: Honoraria, Research Funding; Blackstone Life Sciences: Honoraria; Genentech: Research Funding; Vertex: Other: Spousal employment; TenSixteen Bio: Membership on an entity's Board of Directors or advisory committees, Other: Co-founder; Esperion Therapeutics: Membership on an entity's Board of Directors or advisory committees. Ebert:Exo Therapeutics: Membership on an entity's Board of Directors or advisory committees; Skyhawk Therapeutics: Membership on an entity's Board of Directors or advisory committees; GRAIL: Consultancy; Novartis: Research Funding; TenSixteen Bio: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Deerfield: Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.