Abstract
Background: Ibr and Ven are oral inhibitors of BTK and BCL-2, respectively, approved as single agents or in combination with obinutuzumab for CLL treatment (tx). With distinct and complementary mechanisms of action, Ibr and Ven work synergistically to mobilize CLL cells from lymph nodes and lymphoid niches, enhance cell killing, and eliminate distinct CLL cell populations. CAPTIVATE (NCT02910583) is an international, multicenter phase 2 study evaluating first-line Ibr + Ven in patients (pts) with CLL/SLL who have indication for tx. In this MRD cohort, after completion of Ibr + Ven, pts with Confirmed undetectable minimal residual disease (uMRD) were randomly assigned to placebo (PBO) (ie, a fixed-duration regimen), or continued Ibr. At primary analysis, disease-free survival (DFS) rates were similar in pts from these 2 arms (95% and 100%, respectively), 2 y after randomization (Ghia et al. ASH 2021). Here we present efficacy and safety results with median 56 mo (range, 25-68) follow-up (median 41 mo post randomization).
Methods: Pts aged ≤70 y with previously untreated CLL received 3 cycles of Ibr lead-in then 13 cycles of combined Ibr + Ven (oral Ibr 420 mg/d; oral Ven ramp-up to 400 mg/d). Pts achieving Confirmed uMRD (uMRD serially over at least 3 cycles, in both peripheral blood and bone marrow) with Ibr + Ven were then randomly assigned 1:1 to double-blinded tx with PBO or single-agent Ibr. Endpoints included investigator-assessed best response per iwCLL, rates of uMRD (<10-4 by 8-color flow cytometry), DFS rate (time from randomization to MRD relapse [for a confirmed uMRD pt, ≥10-2 CLL cells/leukocytes confirmed on 2 serial visits], PD per investigator assessment, or death, whichever occurs first), PFS, OS, and AEs.
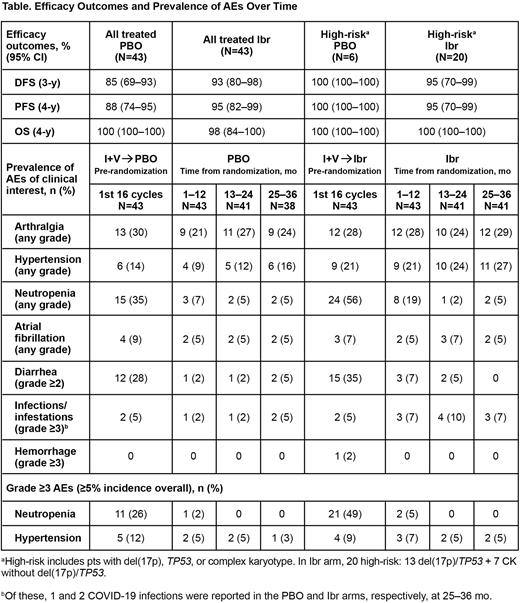
Results: 164 pts were enrolled to receive combined Ibr + Ven tx; after completion, 86 pts with Confirmed uMRD were randomly assigned to PBO or single-agent Ibr (n=43 each). Baseline characteristics were previously reported (Wierda et al. J Clin Oncol. 2021;39:3853). For pts with Confirmed uMRD, median time on study was 56 mo (Ibr arm range, 25‒68 mo; PBO arm range, 40‒65 mo); median post-randomization follow-up was 41.2 mo and 41.5 mo in the PBO and Ibr arms, respectively. In the PBO arm, 63% of pts have now achieved a best response of CR (increased from 60% at 2 y); among pts who continued Ibr, 81% achieved a best response of CR (increased from 72% at 2 y). Rates of uMRD remained stable from y 2 to y 3 post randomization (PBO, 56% [n=24] and 58% [n=25]; Ibr 60% [n=26] and 63% [n=27], respectively). The 3-y DFS rate was 85% (95% CI, 69‒93) with PBO and 93% (95% CI, 80‒98) among pts who continued Ibr (p=0.1621). The 4-y PFS was 88% (95% CI, 74‒95) with PBO and 95% (95% CI, 82‒99) with continued Ibr; 4-y OS was 100% (n=0 deaths) and 98% (95% CI, 84‒100), respectively. Notably, efficacy outcomes in high-risk subgroups were consistent with the total population although low sample size in the PBO arm limits interpretation (Table). Prevalence of AEs during the post-randomization period was generally stable in each arm (Table). New occurrences of hypertension in post randomization ys 1-3 were generally lower with PBO vs Ibr (y 1, n=1/43 vs n=3/43; y 2, n=1/41 vs n=4/41; y 3, n=3/38 vs n=2/41, respectively). No new atrial fibrillation or grade ≥3 hemorrhage events occurred in the PBO arm during the 3-y post randomization period; 1 pt in the Ibr arm had atrial fibrillation in 2nd y post randomization. In the 3rd y post randomization, no pts had dose reduction or discontinuation due to an AE as expected in the PBO arm; 1/41 pts had a dose reduction and 2/41 discontinued Ibr due to an AE. In total, 7 and 2 pts have experienced progressive disease in the PBO and Ibr arms, respectively; 4/7 pts in the PBO arm have initiated subsequent therapy (3 with Ibr, 1 with other agent/s; 0 pts in the Ibr arm have initiated subsequent tx).
Conclusions: First-line Ibr + Ven is an all-oral, once-daily, chemotherapy-free regimen that continues to provide deep, durable clinical responses in pts with CLL. With an additional y of follow-up in pts with Confirmed uMRD after Ibr + Ven, 4-y OS rates were ≥98% and 4-y PFS rates were ≥88% in pts randomly assigned to PBO (representing fixed duration) or continued Ibr. The durability of uMRD and the 3-y DFS rate of 85% without ongoing tx are encouraging and support the promising potential for tx-free remission. Together with the safety data, these results demonstrate a favorable benefit-risk profile with fixed duration Ibr + Ven.
Disclosures
Allan:Genentech: Consultancy, Research Funding; PCYC: Consultancy, Speakers Bureau; BeiGene: Consultancy, Speakers Bureau; TG Therapeutics: Consultancy, Research Funding; Epizyme: Consultancy; ADC Therapeutics: Consultancy; Ascentage: Consultancy; Celgene: Research Funding; Janssen: Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Consultancy, Honoraria, Speakers Bureau. Siddiqi:Beigene: Consultancy, Research Funding, Speakers Bureau; Astrazeneca: Consultancy, Research Funding, Speakers Bureau; Ascentage Pharm: Research Funding; Oncternal: Research Funding; TG Therapeutics: Research Funding; Kite Pharma: Consultancy, Research Funding; BMS: Consultancy; Celgene: Consultancy; Juno Therapeutics: Consultancy, Research Funding; Jannsen: Speakers Bureau; Pharmacyclics: Research Funding, Speakers Bureau. Kipps:Pharmacyclics, LLC an AbbVie Company: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Genentech-Roch: Consultancy, Research Funding; Oncternal: Research Funding; Gilead: Consultancy; Celgene: Consultancy. Kuss:Mundipharma: Consultancy, Honoraria; Roche Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Commonwealth Serum Laboratories: Current equity holder in private company, Current holder of stock options in a privately-held company; AbbVie: Consultancy, Honoraria, Other: expert textinomy, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Merck: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Kyowa Kirin: Consultancy, Honoraria. Badoux:AbbVie: Honoraria, Other: travel, accomodations, expenses; Janssen: Honoraria. Barrientos:Janssen: Honoraria; Beigene: Consultancy; AbbVie: Consultancy; AstraZeneca: Consultancy; MEI: Consultancy; Oncternal: Research Funding; Velosbio: Research Funding; Pharmacyclics, LLC an AbbVie Company: Research Funding. Tedeschi:Beigene: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau. Opat:Pharmacyclics, LLC an AbbVie Company: Research Funding; Antegene: Consultancy, Honoraria, Research Funding; CSL: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; Gilead: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Belgene: Research Funding. Flinn:Genentech: Consultancy, Research Funding; Kite Pharma: Consultancy, Research Funding; Seattle Genetics: Research Funding; Trillium Therapeutics: Research Funding; Genmab: Consultancy; Curis: Research Funding; Forma Therapeutics: Research Funding; Forty Seven: Research Funding; IGM Biosciences: Research Funding; Incyte: Research Funding; Celgene: Research Funding; Constellation Pharmaceuticals: Research Funding; Acerta Pharma: Research Funding; Agios: Research Funding; ArQule: Research Funding; Gilead Sciences: Research Funding; Hutchison MediPharma: Consultancy; Fate Therapeutics: Research Funding; Infinity Pharmaceuticals: Research Funding; Loxo@Lilly: Research Funding; Bristol Myers Squibb: Research Funding; Biopath: Research Funding; CALIBR: Research Funding; CALGB: Research Funding; Iksuda Therapeutics: Consultancy; Nurix Therapeutics: Consultancy, Research Funding; InnoCare Pharma: Consultancy, Research Funding; Vincerx Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Research Funding; Unum Therapeutics: Research Funding; Verastem: Consultancy, Research Funding; MorphoSys: Consultancy, Research Funding; Epizyme: Research Funding; Novartis: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Tessa Therapeutics: Research Funding; Merck: Research Funding; Xencor: Consultancy; Myeloid Therapeutics: Research Funding; Portola Pharmaceuticals: Research Funding; Secura Bio: Consultancy; Rhizen Pharmaceuticals: Research Funding; Roche: Consultancy, Research Funding; Takeda: Consultancy; Servier Pharmaceuticals: Consultancy; Pharmacyclics: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Century Therapeutics: Consultancy; Pfizer: Research Funding; CTI Biopharma: Research Funding; City of Hope National Medical Center: Research Funding; Millenium Pharmaceuticals: Research Funding; TCR2 Therapeutics: Research Funding; 2seventy bio: Research Funding; Triphase Research & Development Corp: Research Funding. Gonzalez Barca:Takeda: Honoraria; Incyte: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; EUSA Pharma: Honoraria, Other: travel, accommodations, expenses; Roche: Honoraria; Janssen: Consultancy, Honoraria, Other: travel, accommodations, expenses; Novartis: Consultancy; Beigene: Consultancy; Gilead: Consultancy; Kiowa: Consultancy; Lilly: Consultancy. Jacobs:TG Therapeutics: Research Funding; Janssen: Speakers Bureau; Beigene: Speakers Bureau; Teneobio: Research Funding; Verastem: Consultancy; MEI Pharma: Research Funding; AstraZeneca: Consultancy, Speakers Bureau; Pharmacyclics, an AbbAvie Company: Consultancy, Research Funding, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau. Szafer-Glusman:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Zhou:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Szoke:Pharmacyclics, an AbbVie Company: Current Employment; AbbVie: Current equity holder in private company, Current holder of stock options in a privately-held company. Wierda:GSK/Novartis: Research Funding; AbbVie: Research Funding; Oncternal Therapeutics: Research Funding; Miragen: Research Funding; Kite Pharma: Research Funding; Bristol Myers Squibb (June and Celgene): Research Funding; Cyclacel: Research Funding; Sunesis: Research Funding; Loxo Oncology/Lilly: Research Funding; Gilead Sciences: Research Funding; AstraZeneca/Acerta Pharma: Research Funding; Pharmacyclics, LLC an AbbVie Company: Research Funding; Janssen: Research Funding; Xencor: Research Funding; Genentech: Research Funding. Ghia:Lilly/Loxo: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria; BMS: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding. Tam:LOXO: Honoraria; AbbVie: Honoraria, Research Funding; Beigene: Honoraria, Research Funding; AstraZeneca: Honoraria; Janssen: Honoraria, Research Funding.
OffLabel Disclosure:
Ibrutinib in combination with venetoclax is not approved in any indication.
Author notes
Asterisk with author names denotes non-ASH members.