Abstract
Purine nucleoside phosphorylase (PNP) converts guanosine to guanine, inosine to hypoxanthine, and 2'-deoxyguanosine to guanine, and leads to the downstream formation of the pro-oxidant and vasculotoxic hypoxanthine and xanthine. Red blood cells are rich in PNP, yet PNP's role in the pathogenesis of sickle cell disease (SCD) is not known. Our group showed that patients with SCD have increased PNP release and activity, leading to accelerated guanosine/inosine metabolism and increased production of vasculotoxic hypoxanthine/xanthine (Bilan VP et al. 2018). Thus, we proposed that PNP may have a major pathogenic role in red blood cell sickling, hemolytic angioproliferation, cell aggregation and end organ damage, and that PNP inhibition may be beneficial in the treatment of SCD.
The highly potent PNP inhibitor 8-aminoguanine is the product of the conversion of the endogenous guanosine metabolite and pro-drug 8-aminoguanosine (8-AG). We investigated the safety and efficacy of short-term (8 weeks) and chronic (20 weeks) treatment of Townes sickle mice (SS) and their non-sickling controls (AA) with 8-AG (60mg/kg/day administered ad libitum in the drinking water). We collected blood and urine samples at multiple intervals using metabolic cages. To determine 8-AG body disposition and PNP inhibition, urine samples were analyzed for purines by liquid chromatography - tandem mass spectrometry (LC-MS/MS).
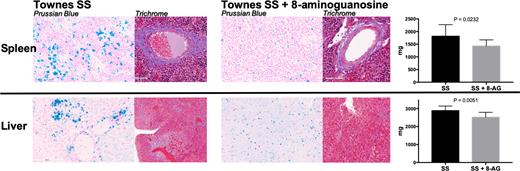
Both AA and SS mice tolerated 8-AG well with no evidence of toxicity. In both AA (n= 15) and SS (n=40) mice there was very efficient conversion of 8-AG that resulted in a 40-50-fold increase in urinary 8-aminoguanine concentrations (p<0.001). In 8-AG treated SS mice we observed reduced hypoxanthine levels, with an increased inosine/hypoxanthine ratio. Additionally, guanosine was increased, guanine was reduced, and an increased guanosine/guanine ratio was observed (p<0.05), suggesting potent PNP inhibition. In SS mice, the 8-week treatment with 8-AG significantly reduced hemoglobinuria (p<0.05) and albuminuria (p<0.05), attenuated left ventricular (LV, p<0.01) and right ventricular (RV, p<0.05) hypertrophy, limited hepatomegaly (p<0.001), and improved RV contractility (p<0.05), with a trend towards reduced lung weights and splenomegaly. In SS mice, 20-week treatment with 8-AG significantly slowed the progression of anemia as evidenced by attenuated age-related drops in hemoglobin and hematocrit levels, and it attenuated hemoglobinuria and albuminuria while significantly reducing splenomegaly (p=<0.05) and hepatomegaly (p<0.05, Figure). Semi-quantitative histopathological analysis suggested reduced iron deposition (Prussian blue stain, Figure), and reduced ischemic (H&E stain) and fibrotic (trichrome stain, Figure) injury in the spleen and liver from SS mice treated with 8-AG.
Our data suggest that the PNP inhibitor 8-AG may have a pharmacological benefit in SCD and may warrant further investigation as a multi-pronged treatment approach for SCD.
Disclosures
Jackson:Deleon Biosciences: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Founder with equity interest; management position; intellectual property rights (e.g., royalties, patents, copyrights) assigned to the University of Pittsburgh and optioned to Deleon Biosciences, Inc., Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.