Abstract
Introduction: Hypercoagulability, thrombosis and microvascular dysfunction are hallmarks of the Philadelphia chromosome-negative myeloproliferative neoplasms (MPN). Elevated peripheral blood cell counts appear to contribute to thrombotic risk but the precise underlying mechanisms remain to be fully elucidated. The interplay between haemostatic and pro-inflammatory pathway activity ('thrombo-inflammation') has emerged in recent years as a source of hypercoagulability in MPN. Platelets are recognised as being mediators of thrombo-inflammation in other diseases and may also be effectors of pro-inflammatory/pro-coagulant activity in MPN. We hypothesized that the platelet proteome is altered in MPN and that its characterisation would reveal insights into the pathophysiology of the disease and the associated thrombotic risk.
Aim: To determine if differences exist in the pattern of platelet protein expression in patients with polycythaemia vera (PV) and essential thrombocythaemia (ET) in contrast to healthy donors.
Methods: 62 patients (ET, n=38; PV, n=24) and 9 healthy volunteers were recruited at Papa Giovanni XXIII Hospital, Bergamo, Italy and the Mater Misericordiae University hospital, Dublin, Ireland. Platelets were isolated from platelet rich plasma, washed in Krebs Ringer buffer and resuspended at 1x 109 platelets/mL in phosphate buffered saline. Whole platelets were lysed in RIPA buffer and differential proteomic signatures established using label-free quantification (LFQ) mass spectrometry (MS), where platelet lysate proteins were double digested using the commercially available PreOmics kit and analysed in a Bruker TimsTOF mass spectrometer connected to a EvoSep liquid chromatography system. Identified peptides were searched against a human FASTA using MaxQuant. For statistical analysis, proteins identified in a minimum of 70% of samples in at least one group were included. Differences in protein expression were determined using an unpaired t-test with a false discovery rate of 5% and a minimal fold change of 0.1 within the Perseus software; p- values below 0.05 were considered significant.
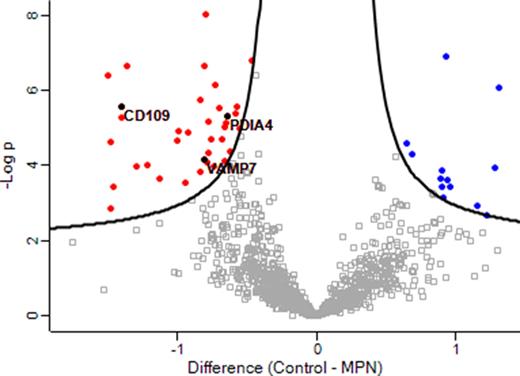
Results: 2,180 proteins from platelet lysates were quantified across all patient and control samples. In MPN platelet lysates, 49 proteins were found to be differentially expressed in comparison to controls (p< 0.05) (Figure 1), including increased expression of markers of platelet activity such as vesicle-associated membrane protein 7 (VAMP-7; regulator of granule exocytosis and actin cytoskeleton activity) and CD109 (a GPI-linked glycoprotein expressed by activated platelets). Bioinformatical analysis revealed a cohort of mitochondrial, cytoskeletal & ribosomal proteins as well as potential effectors of thrombopoiesis and thrombo-inflammation which were significantly upregulated in the MPN cohort. Strikingly, protein disulfide-isomerase (PDI, a member of the thioredoxin superfamily of redox proteins) was increased in MPN lysates. PDI has been shown to contribute to thrombosis, platelet activation and platelet-neutrophil interactions in vivo; plasma levels of PDI have been shown to be increased in MPN and are associated with thrombosis risk.
Conclusion: The platelet proteome is altered in MPN and is suggestive of an activated platelet phenotype with over-expression of proteins with known pro-coagulant activity. To our knowledge the over expression of PDI in MPN platelets has not previously been described. The identification of differentially expressed proteins, such as PDI, provides mechanistic insights into the pathophysiology underlying the substantial burden of arterial and venous thrombosis in MPN and may also assist in the identification of novel therapeutic targets. Additional proteomic analysis of platelet releasate and plasma extracellular vesicles is currently ongoing and may provide additional pathophysiological insights.
Figure 1: Volcano plot of MPN versus control platelet lysate proteomes (x-axis, t-test difference between the mean log2 of the LFQ values; y-axis, the negative log transformed p-value) representing the proteins significantly altered in MPN. Black hyperbolic curves show the threshold for statistical significance. 36 proteins were found to be increased in MPN platelet lysates (including CD109, PDIA4 and VAMP-7) in comparison to control lysates (red) while 13 proteins were decreased in MPN platelet lysates (blue).
Disclosures
Kelliher:Bayer: Other: Conference sponsorship. Ní Áinle:Boston Scientific: Consultancy; Bayer: Research Funding; Daiichi Sankyo: Consultancy, Research Funding. McMullin:Abbvie: Consultancy, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Sierra Oncology: Consultancy; Pfizer: Speakers Bureau; CTI: Consultancy; AOP: Research Funding, Speakers Bureau; BMS: Consultancy, Research Funding; Novartis: Consultancy, Speakers Bureau. Conneally:Jazz: Honoraria; Abbvie: Membership on an entity's Board of Directors or advisory committees; Astellas: Honoraria; Novartis: Honoraria. Maguire:Bayer: Research Funding; Mallinckdrodt: Research Funding; Sanofi S.A.: Research Funding; Intel Ireland: Research Funding; Google: Research Funding. Kevane:Bayer: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.