Abstract
Introduction: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, life-threatening disease characterized by complement-mediated hemolysis and thrombosis. Pegcetacoplan (PEG) is the first approved C3 inhibitor for US adults with PNH and EU adults with PNH who are anemic after treatment with a C5 inhibitor for ≥3 months. In 5 clinical trials, PEG significantly improved hemoglobin (Hb) and other disease parameters in patients with PNH who were anemic, despite eculizumab (ECU) treatment, or who were naïve to complement inhibitors. We present 48-week data from our ongoing extension study (study 307; NCT03531255) designed to evaluate the long-term efficacy and safety in patients previously enrolled in PEG clinical trials.
Methods: This open-label, multicenter extension study enrolled adults with PNH who completed previous PEG phase I, II and III trials. Patients from PHAROAH (Phase 1) and PEGASUS (Phase 3) had baseline Hb <10.5 g/dL despite ≥3 months of stable ECU dosing. Over a third of PEGASUS patients entered the study on higher-than-approved doses of ECU, with high transfusion burden, and were chronically anemic. Patients from PADDOCK (Phase 1b), PALOMINO (Phase 2a), and PRINCE (Phase 3) trials were complement inhibitor-naïve at baseline. The current study continued patients on their current PEG dose (1080 mg subcutaneous [SC] twice weekly or every 3 days, PEGASUS and PRINCE) or switched patients to 1080 mg SC PEG treatment (PHAROAH, PADDOCK, PALOMINO). Efficacy was evaluated with mean hematologic values (Hb, lactate dehydrogenase [LDH]), Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score, and transfusion avoidance (i.e., patients [%] who did not require transfusion during the 48 weeks; patients who withdrew did not meet transfusion avoidance criteria). The proportion of patients with normalized Hb and LDH levels was determined. Post hoc analyses determined mean change from baseline to Week 48 for Hb, LDH, and FACIT-Fatigue scores. Safety was assessed by evaluating the incidence of adverse events (AEs).
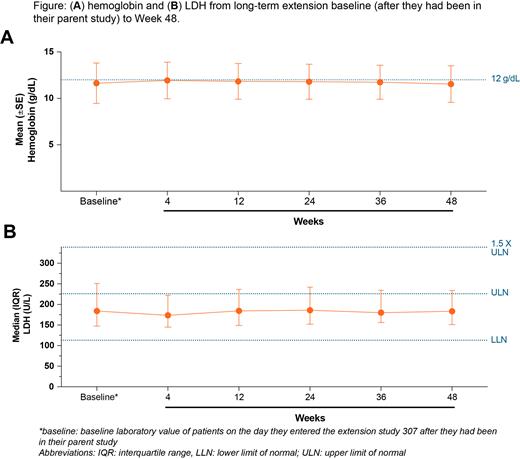
Results: A total of 137 of 145 patients who completed a previous PEG trial chose to enter the extension study and 107 had received 48 weeks of treatment at the time of data cutoff in addition to the treated time in the parent study. Total 1080 mg twice weekly PEG exposure ranged from 74 weeks (PRINCE) to 96 weeks (PEGASUS). At baseline, mean (SD; median) Hb was 11.6 g/dL (2 g/dL; 11.8 g/dL). At week 48, Hb was also 11.6 g/dL (1.9 g/dL; 11.7 g/dL), and 35.1% of patients had normal Hb per sex-specified norms. Baseline mean (SD; median) LDH was 284.2 U/L (380 U/L; 181.5 U/L). At week 48, mean (SD; median) LDH was 274.2 U/L (319.1 U/L; 180.1 U/L), and 73.2% of patients had normalized LDH levels. Baseline mean (SD; median) FACIT-fatigue was 42.8 (8.8; 46.0). At week 48, mean (SD; median) FACIT-fatigue was 42.4 (9.8; 45.0). Baseline Hb, LDH, and FACIT-fatigue scores were well maintained throughout the study (Figure). Transfusion avoidance was achieved in 83.2% of patients through Week 48.
Most patients had an AE (73.7%) through Week 48; 16.1% of AEs were judged by investigators to be related to PEG. The most common treatment-emergent AE was hemolysis, reported in 16.8% of patients (14/64 PEGASUS patients, 6/50 PRINCE patients). Serious AEs (SAEs) were reported in 19.7% of patients. No SAEs were judged related to PEG. The most commonly reported SAE was hemolysis (8% of patients). Of the 11 patients with an SAE of hemolysis, 9 were from the PEGASUS study. Seven of these 9 patients received an increased PEG dose (2 of the 7 also received an ECU dose), and only 3 required transfusions. All serious hemolytic events resolved. Injection site reactions occurred in 10.9% of patients; most were mild and transient. No thrombotic events or meningitis infections were reported. Three patients discontinued due to hemolysis. One death occurred (sudden cardiac event); it was deemed unrelated to PEG.
Conclusions: These long-term results show that pegcetacoplan treatment sustains robust improvements in Hb, LDH and fatigue in patients with PNH, and reduces the need for transfusions. Normalization of hematologic parameters is achievable with pegcetacoplan. The long-term safety data corroborate the favorable safety profile reported in previous clinical trials. An evaluation of the safety and efficacy of intensive subcutaneous or intravenous dosing of pegcetacoplan in order to treat acute hemolytic events is underway.
Disclosures
Patriquin:Alexion: Consultancy, Honoraria; Apellis: Consultancy, Honoraria; Regeneron: Consultancy; Takeda: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; Sobi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria. Bogdanovic:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda Pharmaceutical Company: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Membership on an entity's Board of Directors or advisory committees; Apellis Pharmaceuticals: Other: Investigator fee in clinical trial. Griffin:BioCryst Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Alexion: Honoraria, Other: Conference support; Sobi Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Medscape: Other: educational work sponsored by Apellis with unrestricted grant paid to Medscape. Kelly:Biocryst: Membership on an entity's Board of Directors or advisory committees; Swedish Orphan Biovitrum AB: Membership on an entity's Board of Directors or advisory committees; Medscape: Other: educational work sponsored by Apellis with unrestricted grant paid to Medscape; Sobi: Research Funding; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: conference support; Novartis: Membership on an entity's Board of Directors or advisory committees; Biologix: Research Funding; Jazz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Astellas: Research Funding; Amgen: Consultancy. Maciejewski:Apellis Pharmaceuticals: Consultancy; Alexion: Consultancy. Roeth:Sanofi: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Roche: Consultancy, Honoraria, Research Funding; Biocryst: Consultancy, Honoraria; Apellis Pharmaceuticals: Consultancy, Honoraria; Alexion Pharmaceuticals: Consultancy, Honoraria, Research Funding. Selvaratnam:Apellis Pharmaceuticals: Consultancy. Szer:Novartis: Consultancy, Honoraria, Speakers Bureau; Alexion: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda Pharmaceuticals: Honoraria, Speakers Bureau; Pfizer: Honoraria, Speakers Bureau; Prevail Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Apellis: Consultancy. Savage:Apellis Pharmaceuticals: Current Employment. Horneff:Swedish Orphan Biovitrum AB: Current Employment. Tan:Swedish Orphan Biovitrum AB: Current Employment. Yeh:Apellis Pharmaceuticals: Current Employment, Current equity holder in publicly-traded company. Panse:Apellis Pharmaceuticals: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Grunenthal: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Speakers Bureau; Chugai: Speakers Bureau; Blueprint Medicines: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.