Abstract
Background: Existing clinical and biological features suboptimally capture risk of DLBCL treatment failure after curative intent first-line (1L) therapy. PhasED-Seq improves the analytical sensitivity of minimal residual disease (MRD) detection by two orders of magnitude over current circulating tumor DNA (ctDNA) commercial methods in use today (Chen et al. 2021 Mol Diag). We hypothesized that ultrasensitive ctDNA-based MRD detection by PhasED-Seq can accurately track response and predict curative outcomes after experimental 1L therapy.
Methods: We profiled 82 serial blood specimens (68 plasma and 14 peripheral blood mononuclear cell (PBMC) samples) from evaluable patients enrolled in a randomized Phase Ib study in de novo DLBCL treated with Tafasitamab in combination with Lenalidomide & R-CHOP (T/LR-CHOP: firstMIND; NCT04134936). Cell-free DNA was profiled by PhasED-Seq (Kurtz et al. 2021 Nat Biotech) in the Foresight Diagnostics CLIA laboratory (Aurora, CO). Pretreatment plasma and PBMC specimens were used to noninvasively genotype each patient's tumor to identify Phased Variants (PVs), and these were used to monitor MRD in subsequent blood specimens (n=68), collected at C2D1, C4D1, End-of-Therapy (EOT), and six-month follow-up. Patients/samples were reported as MRD positive when ctDNA levels exceeded an analytical detection threshold (~1 part per million cfDNA molecules), corresponding to 98% clinical specificity.
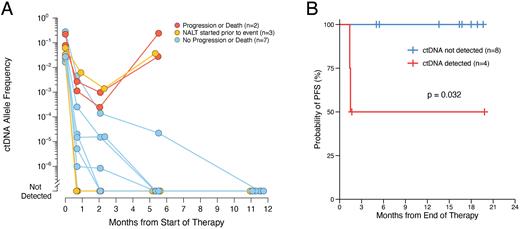
Results: Among 66 subjects from NCT04134936, 50% were randomized to T/LR-CHOP therapy. Among T/LR-CHOP treated patients (n=33), patients with (n=14, 42%) or without (n=19, 58%) available MRD specimens were similar in age, stage, IPI, and cell-of-origin subtype (p=N.S.). ctDNA profiling of additional evaluable subjects from the full T/L cohort is ongoing and expanded results will be presented. In the absence of tumor tissue, 12 out of 14 patients had sufficient ctDNA to enable identification of PVs for monitoring MRD using pre-treatment plasma. Pretreatment ctDNA abundance (median=454 hGE/mL) was similar to other DLBCL cohorts tested by PhasED-Seq. Pretreatment levels correlated with LDH levels (p<0.001). T/LR-CHOP therapy induced MRD-negativity rates of 25%, 50%, and 67% after 1 cycle (C2D1), 3 cycles (C4D1), and 6 cycles (EOT), respectively. MRD-positivity at each timepoint was associated with likelihood of future progression or death, achieving positive predictive values of 25%, 33%, and 67% at C2D1, C4D1, and EOT, respectively (Fig. A). MRD-positivity at EOT was significantly associated with PFS (p=0.03; Fig. B), and none of the patients who were MRD-negative at EOT have developed disease progression.
Conclusions: Collectively, these results demonstrate that absence of ctDNA MRD using an ultrasensitive approach strongly correlates with durable responses to T/LR-CHOP and could potentially be used in the future as a surrogate for clinical benefit, including in the randomized phase trial of this combination (frontMIND; NCT04824092).
Figure. The ctDNA MRD Assay PhasED-Seq Predicts Progression-free Survival after T/LR-CHOP in DLBCL. (A) Spiderplot depicts ctDNA levels for 12 subjects before and after T/LR-CHOP. (B) Kaplan-Meier plot shows that ctDNA detection by PhasED-Seq at the end of therapy (EOT) stratifies patients according to probability of progression-free survival (PFS).
Disclosures
Kurtz:Foresight Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties; Adaptive Biotechnologies: Consultancy; Roche: Consultancy; Genentech: Consultancy. Hogan:Foresight Diagnostics: Current Employment; Freenome: Other: Equity holder. Schultz:Foresight Diagnostics: Current Employment, Current holder of stock options in a privately-held company. Kopeckova:Novartis: Current equity holder in publicly-traded company, Honoraria; Laboratoires Pierre Fabre: Current equity holder in private company; Viatris: Current equity holder in publicly-traded company; EISAI: Research Funding. Kuffer:MorphoSys AG: Current Employment, Current equity holder in publicly-traded company. Blair:BMS: Current equity holder in private company; MorphoSys, AG. Inc: Current Employment. Wagner:MorphoSys: Current Employment, Current holder of stock options in a privately-held company, Other: Travel and accomodation expenses. Close:Foresight Diagnostics: Current Employment, Current holder of stock options in a privately-held company. Diehn:Foresight Diagnostics: Consultancy, Current equity holder in private company. Chabon:Foresight Diagnostics: Current Employment, Current holder of stock options in a privately-held company. Alizadeh:Genentech: Consultancy; Adaptive Biotechnologies: Consultancy; Gilead: Consultancy, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties; Syncopation: Current equity holder in private company, Patents & Royalties; Cibermed Inc: Consultancy, Current equity holder in private company, Patents & Royalties; Foresight Diagnostics: Consultancy, Current equity holder in private company, Patents & Royalties; BMS: Consultancy, Research Funding; Roche: Consultancy; Karyopharm: Consultancy. Westin:Bristol Myers Squibb: Consultancy, Research Funding; ADC Therapeutics: Consultancy, Research Funding; MorphoSys/Incyte Corporation: Consultancy, Research Funding; Abbvie/GenMab: Consultancy; Novartis: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Genentech/Roche: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy, Research Funding; MonteRosa: Consultancy; Calithera: Consultancy, Research Funding; Iksuda: Consultancy; Merck: Consultancy; SeaGen: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.