Abstract
Introduction The United States (US) Food and Drug Administration has recently approved chimeric antigen receptor (CAR) T-cell therapy for patients with relapsed/refractory mantle cell lymphoma (MCL) and follicular lymphoma (FL). Although CAR T-cell therapy is commonly administered in inpatient settings, as clinicians gain experience, there is growing interest in providing infusions and follow-up care in the outpatient setting. This study describes patient characteristics, treatment setting, CAR T-cell therapy-associated adverse events (CAR T-AEs), and health resource utilization (HRU) for real-world CAR T-cell therapy use for MCL and FL.
Methods A retrospective analysis of the Anlitiks All-Payor Claims (AAPC) data for services rendered from April 2017 to March 2022 was conducted. The database includes open-source fully adjudicated pharmacy and medical claims of patients who are insured through Medicare, Medicaid, or commercial plans, representing over 80% of the US population. MCL (ICD-9/10-CMs: 200.x, C83.1x) and FL patients (ICD-9/10-CMs: 202.xx, C82.xx) with a first claim (index date) for CAR T-cell therapy (MCL: brexucabtagene autoleucel [brexu-cel] or unspecified CAR T-cell agent; FL: lisocabtagene maraleucel [liso-cel], tisagenlecleucel [tisa-cel], axicabtagene ciloleucel [axi-cel] or unspecified agent) between October 2017 and December 2021, with ≥ 180 days of pre-index and ≥ 90 days of post-index follow-up were identified. Demographics, clinical characteristics, and treatment (infusion) setting (inpatient/outpatient Authorized Treatment Centers [ATCs]) were examined. Incidence of observed CAR T-AEs and HRU were analyzed overall and stratified by infusion setting. Descriptive statistics were reported as frequencies and percentages for categorical variables; mean, median, and range for continuous variables. Chi-square tests (categorical measures), t-tests, and Wilcoxon-rank sum tests (continuous measures) were used to assess group differences, where appropriate. Time from leukapheresis to CAR T-cell infusion was assessed with adjusted Cox proportional hazards models.
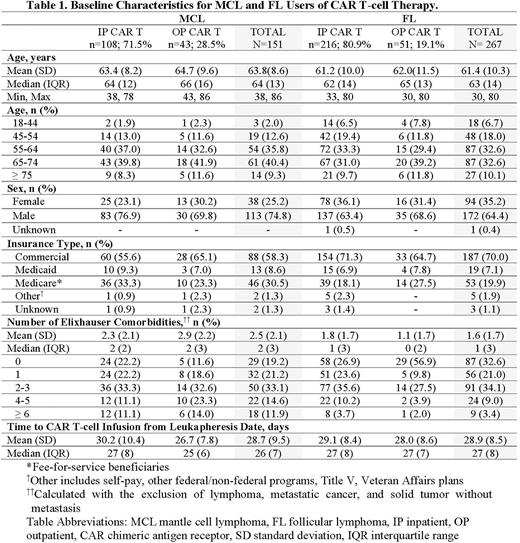
Results The final sample consisted of a total of 151 MCL and 267 FL patients treated with CAR T-cell therapy. Among MCL patients, 75% were male, mean age was 63.8 (±8.6) years, and 58.3% (n=88) were commercially insured while 30.5% (n=46), 8.6% (n=13) and 2.6% (n=4) had Medicare, Medicaid and unknown/other insurance types, respectively. Among FL patients, 65% were male, mean age was 61.4 (±10.3) years, and 70.0% (n=187) were commercially insured while 19.9% (n=53), 7.1% (n=19) and 3.0% (n=8) had Medicare, Medicaid and unknown/other insurance types, respectively. Overall, 71.5% and 80.9% of MCL and FL patients were infused inpatient, respectively. Among FL patients with a specified CAR T-cell therapy, axi-cel accounted for the majority of patients. Median time from leukapheresis to infusion was similar for inpatient (MCL/FL: 27 days) and outpatient (MCL: 25 days; FL: 27 days) settings. Both MCL brexu-cel and FL axi-cel patients had similar median time from leukapheresis to infusion compared to their respective unspecified CAR T patient cohorts (MCL: both 26 days; FL 25 and 27 days). A total of 113 (74.8%) and 182 (68.2%) MCL and FL patients had an observed CAR T-associated AE within 90 days post-infusion, respectively. HRU analysis is underway.
Conclusions In this analysis, nearly 3 in 4 MCL patients and more than 4 in 5 FL patients received CAR T-cell therapy infusions in inpatient settings. Median time from leukapheresis to CAR T-cell infusion was similar for MCL and FL in both infusion settings. Approximately three-fourths of MCL patients and two-thirds of FL patients experienced at least one observed CAR T-associated AE within 90 days post-infusion. Recent data on real-world treatment patterns enhance understanding of CAR T-cell therapy use in the evolving MCL and FL treatment landscape.
Disclosures
Seyedin:Kite, A Gilead Company: Consultancy. Hasegawa:Kite, A Gilead Company: Current Employment. Rajagopalan:Kite, A Gilead Company: Consultancy. Wade:Abbvie: Consultancy; Kite, A Gilead Company: Consultancy; Johnson & Johnson: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.