Abstract
Introduction: Despite most patients with cHL being cured with standard therapies, a proportion of patients are refractory or relapse after first- and second-line treatments (1L/2L), including stem cell transplantation, and there are limited treatment options available after failure of brentuximab vedotin (BV) and PD-1 blockade. Camidanlumab tesirine (Cami) is an antibody-drug conjugate comprising an anti-CD25 monoclonal antibody conjugated through a cleavable linker to a pyrrolobenzodiazepine (PBD) dimer. Cami has shown notable single-agent anti-tumor activity and manageable toxicity in the Phase 2 trial of patients with R/R cHL (Carlo-Stella et al, Hemasphere 2022;6:102-103).
Objectives: To assess the clinical response and safety of Cami by subgroups based on demographics, known risk factors impacting outcomes in patients with R/R cHL, and factors with potential relevance to Cami's mechanism of action.
Methods: This analysis was based on the open-label, multicenter, Phase 2 study of Cami monotherapy in patients with R/R cHL after ≥3 prior lines of therapy (NCT04052997). Cami was administered (30-minute infusion) on day 1 of each 3-week cycle at 45 µg/kg for 2 cycles, then 30 µg/kg for subsequent cycles. Subgroup analyses (data cutoff: March 16, 2022) were conducted (Table 1). Efficacy outcomes included the overall response rate (ORR) and median duration of response (mDOR); statistical significance was assessed by comparison of 95% confidence intervals. Safety was assessed by incidence of treatment emergent adverse events (TEAEs).
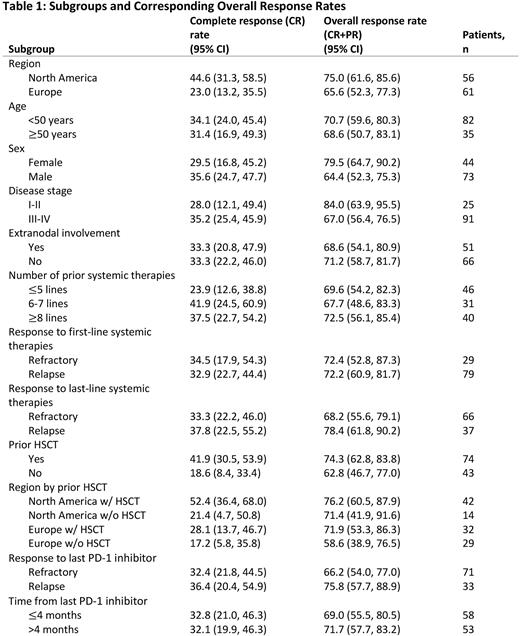
Results: There were no significant differences in efficacy outcomes between demographic subgroups based on age or sex (Table 1). The ORR was similar for patients who were refractory or relapsed after 1L therapy (72.4% vs 72.2%). Despite the higher number of patients who were refractory after last line therapy versus those who relapsed, the ORR was not significantly different between the two groups (68.2% vs 78.4%). The response to Cami did not depend on the prior response to PD-1 inhibition or the time since the last PD-1 inhibitor use. The ORR was similar for patients who were refractory to PD-1 inhibition or relapsed (66.2% vs 75.8%) and for patients who were treated with Cami ≤4 months and >4 months since the last PD-1 inhibitor (69.0% vs 71.7%).
No statistically significant differences in the ORR were observed based on the number of prior lines of therapy, prior HSCT, or region. However, the complete response rate (CRR) for patients who received prior HSCT was 41.9% vs 18.6% in patients without HSCT; the mDOR was 13.73 months in patients with prior HSCT vs 5.85 in patients without prior HSCT. Median DOR was similar between North American (NA) and European (EU) patients (13.77 months vs 13.73 months). However, NA patients had improved CRR vs EU patients (44.6% vs 23%), and differences were more pronounced in NA vs EU patients with prior HSCT (52.4% vs 28.1%).
Cami's tolerability was similar across most subgroups. The safety profile of Cami was similar in older compared with younger patients, as indicated by similar incidences of TEAEs overall, grade ≥3 TEAEs, and groupings by system organ class. The incidence of any grade TEAEs was similar between NA and EU patients (100% vs 98.4%). Patients with prior HSCT had more grade ≥3 TEAEs vs those without prior HSCT (73% vs 58.1%).
Conclusions: Evidence from preliminary subgroup analyses suggests that response to Cami was independent of age, sex, and response to and timing of last PD-1 inhibitor. Differences in Cami's safety and efficacy were noted by prior transplant and region. Although a difference in CRR was observed between NA and EU, this did not result in a difference in mDOR or ORR between the two regions. These preliminary analyses should be interpreted with caution as subgroups were small and limited in statistical comparison. Exploratory analyses to identify predictive markers of GBS/polyradiculopathy observed in Cami trials are ongoing and results will be also presented. These results suggest that Cami shows antitumor activity across patient subgroups, including heavily pretreated and older patients.
Funding: ADC Therapeutics SA; medical writing: CiTRUS Health Group.
Disclosures
Herrera:Takeda: Consultancy; KiTE Pharma: Research Funding; Regeneron: Consultancy; Caribou: Consultancy; Tubulis: Consultancy; Pfizer: Consultancy; Genmab: Consultancy; Genentech: Consultancy, Research Funding; Merck: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Adicet Bio: Consultancy; ADC Therapeutics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Gilead: Research Funding; Karyopharm: Consultancy. Ansell:SeaGen: Research Funding; Takeda: Research Funding; Bristol Myers Squibb: Research Funding; Regeneron: Research Funding; Affimed: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Research Funding. Zinzani:Kyowa Kirin: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Eusapharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; University of Bologna: Current Employment. Radford:Kite Pharma: Consultancy; Astrazenca: Current equity holder in private company, Current holder of stock options in a privately-held company; ADC Therapeutics: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; The University of Manchester and Christie Hospital NHS Foundation Trust: Current Employment. Maddocks:ADC Therapeutics: Consultancy; Acerta: Consultancy; AstraZeneca: Consultancy; Beigene: Consultancy; Celgene: Consultancy; Genmab: Consultancy; Morphosys: Consultancy; Lilly: Consultancy; Janssen: Consultancy; Kite: Consultancy; Incyte: Consultancy; Gilead: Consultancy; Genentech: Consultancy; Abbvie: Consultancy; BMS: Consultancy, Research Funding; Pfizer: Research Funding; Pharmacyclics: Consultancy, Research Funding. Pinto:F. Hoffmann-La Roche AG: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier Affaires Medicales: Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck Sharp and Dohme: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees. Collins:SecuraBio: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants / expenses, Speakers Bureau; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants / expenses, Speakers Bureau. Bachanova:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; FATA Therapeutics: Research Funding; Incyte: Research Funding; Gamida Cell: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharma: Consultancy; Citius Pharma: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Bartlett:Washington University School of Medicine: Current Employment; Autolus, Bristol-Meyers Squibb, Celgene, Forty Seven, Janssen, Kite Pharma, Merck, Millennium, Pharmacyclics: Research Funding; ADC Therapeutics, Roche/Genentech, Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Hamadani:Medical University of Wisconsin: Current Employment; MorphoSys: Consultancy; Incyte Corporation: Consultancy; Kite: Consultancy; Omeros: Consultancy; Legend Biotech: Consultancy; ADC Therapeutics: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy; Kadmon: Consultancy; Gamida Cell: Consultancy; SeaGen: Consultancy; Genmab: Consultancy; Abbvie: Consultancy; Takeda: Research Funding; Spectrum Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; Sanofi Genzyme: Speakers Bureau; AstraZeneca: Speakers Bureau; BioGene: Speakers Bureau. Kline:iTeos, Merck, Verastem: Research Funding; Karyopharm, Kite/Gilead, Merck, MorphoSys, Seagen, Verastem: Consultancy. Mayer:MSD: Research Funding. Savage:BMS, Janssen, Kyowa, Merck, Novartis, and Seattle Genetics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Beigene and Regeneron: Membership on an entity's Board of Directors or advisory committees. Advani:ADC Therapeutics, Cyteir, Daiichi Sankyo, Gilead, Merck, Regeneron, Roche, Seattle Genetics: Research Funding; ADC Therapeutics, BMS, Daiichi Sankyo, Epizyme, Gilead, Incyte, Merck, Roche, Sanofi: Consultancy. Caimi:Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; MEI Pharma: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees. Casasnovas:Roche, Gilead, Takeda: Research Funding; Roche, Takeda, BMS, MSD, Gilead/Kite, Janssen, ADC Therapeutics, Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Roche, Takeda, Merck, BMS, Gilead/Kite, Abbvie, ADC therapeutics, INCYTE, AstraZeneca: Honoraria. Hess:AstraZeneca: Consultancy, Speakers Bureau; Bristol-Myers Squibb: Consultancy; ADC Therapeutics: Consultancy. Bastos-Oreiro:KITE/GILEAD: Consultancy, Honoraria; NOVARTIS: Speakers Bureau; INCYTE: Consultancy, Speakers Bureau; JANSSEN: Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau. Iyengar:Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Speaker Fees and conference support; Lilly: Membership on an entity's Board of Directors or advisory committees; Kite: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Janssen: Other: Speaker fees; Abbvie: Other: conference support. Szomor:Takeda: Honoraria; Roche: Honoraria; Abbvie: Honoraria; Novartis: Honoraria; Janssen: Honoraria. Townsend:Takeda, Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria. Andre:Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche: Other: Travel Grant , Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grant; Johnson & Johnson: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; Abbvie: Other: Travel Grant; Astra Zenca: Other: Travel Grant; BRS: Membership on an entity's Board of Directors or advisory committees, Other: Travel Grant; Celgene: Other: Travel Grant. Dyczkowski:ADC Therapeutics: Consultancy. Eisen:ADC Therapeutics: Consultancy. Urban:ADC Therapeutics: Consultancy. Pantano:Pembrolizumab: Ended employment in the past 24 months; Alcon: Current equity holder in publicly-traded company; Novartis: Current equity holder in publicly-traded company, Other: Spouse works at Novartis; Merck: Current equity holder in publicly-traded company; Organon: Current equity holder in publicly-traded company; ADC Therapeutics SA: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Cruz:ADC Therapeutics SA: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Wang:ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Negievich:ADC Therapeutics SA: Current Employment; ADC Therapeutics: Current equity holder in private company, Current holder of stock options in a privately-held company; Imugene: Current equity holder in publicly-traded company; Seattle Genetics: Current equity holder in publicly-traded company. Wuerthner:Scenic Biotech: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Carlo-Stella:Roche: Other: Consultancy/Advisory, Research Funding; Sanofi: Other: Consultancy/Advisory, Research Funding; Bristol Myers Squibb: Honoraria; Merck Sharp & Dohme: Honoraria; Janssen Oncology: Honoraria; AstraZeneca: Honoraria; Incyte: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Celgene/Bristol Myers Squibb: Other: Consultancy/Advisory; Karyopharm Therapeutics: Other: Consultancy/Advisory; Scenic Biotech: Other: Consultancy/Advisory; ADC Therapeutics: Honoraria, Other: Consultancy/Advisory, Research Funding.
OffLabel Disclosure:
Camidanlumab tesirine is an investigational agent.
Author notes
Asterisk with author names denotes non-ASH members.