Abstract
Introduction. The Australasian Leukaemia and Lymphoma Group (ALLG) MDS4 phase II trial randomized 160 patients (pts) with higher risk (by IPSS) myelodysplastic syndromes (MDS), chronic myelomonocytic leukemia (CMML) and low blast acute myeloid leukemia (AML) to either azacitidine (AZA) or combination AZA with lenalidomide (LEN, from cycle 3 onwards) (Kenealy, Haematologica 2019; ANZCTR12610000271000). Whilst the combination of LEN and AZA was tolerable, there was no improvement in clinical benefit, response rates or overall survival in patients compared to treatment with AZA alone. We performed a post-hoc genomic analysis of pts on this trial to further understand the molecular changes in this cohort and any correlation with outcomes.
Methods. A unique molecular index (UMI) based QIAseq targeted DNA panel (QIAGEN) was performed as previously described (Blombery, Blood 2020). Sensitivity for variant calling was 0.5% variant allele frequency (VAF). HumanCytoSNP-12 BeadChip array (SNP-A) was previously performed and reported (Kenealy, Haematologica 2019). Primary endpoint of the study was ongoing clinical benefit at 12 months, defined as being alive and progression-free. Logistic regression and Cox proportional hazards regression were used respectively to assess the differences in response rates and overall survival between two treatment groups according to the molecular subgroups.
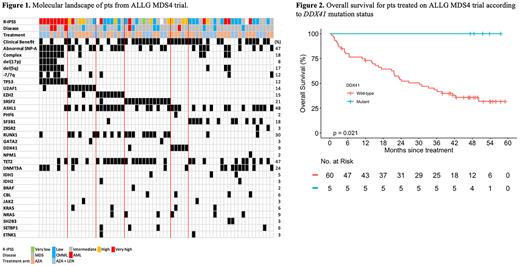
Results. A total of 66 pts, representative of the 160 enrolled, had baseline gDNA available for targeted panel testing; 36 and 30 pts on AZA alone and AZA+LEN therapy, respectively. Median age was 71.6 years. IPSS-R risk groups were very low or low (n=20), intermediate (n=18), high or very high (n=25), and not available (n=3 due to missing karyotype). Disease subtypes were MDS (n=52), CMML (n=9), and AML (n=5). 47% of pts had an abnormal SNP-A, including 18% with complex (≥3) copy number changes. The most common gene mutations observed were ASXL1 (48.5%), TET2 (47%) and RUNX1 (30%) (Figure 1).
Overall clinical benefit at 12 months was achieved in 39 pts (59%). TET2 was the only gene mutation associated with significantly improved benefit when treated with AZA alone (OR 10 [95% CI, 2 to 81]). Median follow up among survivors was 49 months and the median overall survival was 33.2 months (95% CI, 21.7 - NE). Mutations in TP53, U2AF1, EZH2 and SRSF2 were associated with inferior overall survival. Molecular subgroup analyses did not identify any significant difference between the two treatment arms.
Given the recent discovery of DDX41 mutations in MDS/AML and early data supporting a favorable response to AZA/LEN, we focused on 6/66 (9%) patients with detectable DDX41 mutations. All 6 pts had a second mutation detectable in DDX41 as well as no detectable abnormalities on SNP-A. The most commonly co-mutated gene in this group was ASXL1 (in 4/6). One patient with DDX41 mutation died before receiving any therapy. Best responses achieved in DDX41 mutated patients were complete remission (CR, n=1), marrow CR (n=1), hematologic improvement (HI, n=1), and stable disease (SD, n=2). Remarkably, of the 5 patients that received treatment (4 AZA, 1 AZA+LEN), the overall survival at 52 months (range 47-58) follow up was 100% (Figure 2), despite having intermediate (n=3) and high (n=2) R-IPSS.
Mutations were also serially assessed on therapy in 45 pts: ≤2 cycles in 38 pts, and ≥4 cycles in 7 pts (2 AZA+LEN). Of 166 mutations observed at baseline, majority (67%) were stable with <10% change in VAF (median +2%). Overall, 27 (60%) pts had clearance or >10% VAF reduction in ≥1 variant, including clearance in 20 (12%) variants (most commonly TET2 [n=4] and RUNX1 [n=3]), and reduction in 33 (20%) variants (ASXL1 [n=6], SF3B1, SRSF2 and TET2 [n=5 each], but no pt had complete molecular clearance. In contrast, new mutations (n=18 variants) or >10% VAF increase (n=2 variants) were observed in 14 (31%) pts, most commonly TET2 (n=6), RUNX1 (n=4), and DNMT3A and BRAF (n=3 each).
Conclusions. In summary, our correlative molecular analysis of the ALLG MDS4 trial of AZA vs AZA+LEN in MDS/AML has identified, in addition to established molecular risk factors for inferior outcomes, that pts with DDX41 mutations are associated with highly favorable outcomes with either AZA or AZA+LEN. This observation and subgroup of patients warrants further study in randomized trials.
Disclosures
Tiong:Pfizer: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Servier: Consultancy, Speakers Bureau. Seymour:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genor Biopharma: Membership on an entity's Board of Directors or advisory committees. Blombery:Adaptive Biotechnologies: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria; Servier: Honoraria.
OffLabel Disclosure:
This presentation will discuss the use of lenalidomide in higher risk MDS and low blast count AML
Author notes
Asterisk with author names denotes non-ASH members.