Abstract
Introduction: Treatment of chronic lymphocytic leukemia (CLL) has been revolutionized by the introduction of Bruton Tyrosine Kinase inhibitors (BTKi). Ibrutinib (IBR) and acalabrutinib (ACA) are two BTKi approved for the treatment of CLL patients (pts) with both frontline and relapsed disease. Based on its kinome inhibitory properties, IBR has more off-target kinase interactions compared to the second-generation BTKi ACA, including inhibition of ITK, HER2, HER4, and TEC, likely leading to increased off-target toxicity (Estupiñán, 2021 and McMullen, 2014). Results from the first randomized phase III trial comparing ACA to IBR in pts with previously treated CLL found a lower incidence of atrial fibrillation (Afib) with ACA (Byrd, 2021). However, there is lack of information on tolerability of ACA in pts treated outside clinical trials who had received prior IBR treatment. Our study aimed, therefore, to evaluate the real-world tolerability of ACA after IBR therapy in pts with CLL with specific attention to cardiovascular effects.
Methods: All pts with CLL treated at our institution between 11/2019 and 5/2021 with ACA after prior IBR therapy were included in this study. Pts with less than 6 months of follow up were excluded unless they died prior to that period. Pts with confirmed Richter's Transformation were also excluded. All data were retrospectively obtained through institutional electronic health records, including demographics, clinical and genetic prognostic factors, toxicities, survival outcomes, duration of ACA therapy and reasons for IBR and ACA discontinuation. All grading of adverse events was completed utilizing the Common Terminology Criteria for Adverse Events (CTCAE), International Society on Thrombosis and Hemostasis (ISTH), or European Organization for Research and Treatment of Cancer (EORTC) definitions, as appropriate. All adverse events considered possibly related to BTKi are reported. A waiver of informed consent was granted for this retrospective study.
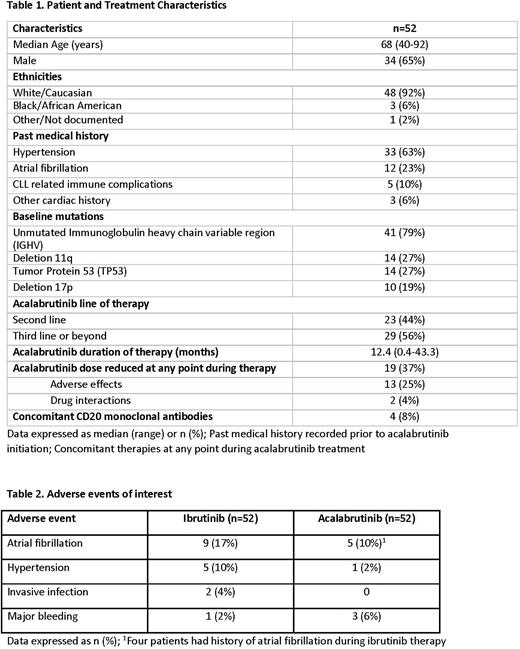
Results: 52 pts treated with ACA and history of prior IBR treatment were identified (Table 1). The median age was 68 years (range, 40-92 years) and 25% were > 75 years old. ACA was administered for a median of 12.4 months (range, 0.4-43.3 months) with 19 pts (37%) requiring a dose reduction at some point during ACA treatment. The most commonly reported adverse effects during IBR treatment included arthralgia/myalgia (31%), Afib (17%), rash (15%), GI upset (12%), and hypertension (HTN, 10%). The reason for IBR treatment discontinuation was adverse effects in 47 pts (90%), progression in 4 pts (8%) and pts preference in 1 (2%). The most commonly reported adverse effects during ACA treatment included GI upset/GERD (25%), headache (17%), arthralgia/myalgia (12%), Afib (10%) and cytopenias (10%). At the time of data collection, 17 (33%) patients had discontinued ACA treatment. The reason for ACA treatment discontinuation was adverse effects in 14 pts (27%), progression in 2 pts (4%) and pt preference in 1 (2%). Specific attention was given to cardiovascular effects since a significant proportion of pts had a past medical history of cardiac comorbidities prior to ACA treatment, including history of HTN in 33 pts (63%) and Afib in 12 pts (23%). New onset Afib developed in 1 pt (2%) and recurrent Afib was observed in 4 pts (8%) during ACA therapy. The 4 pts with recurrent Afib had history of Afib while on IBR therapy. Median time to Afib events with ACA was 4.4 months (range, 0.5-13.3 months). New or worsening HTN developed in only 1 pt while on ACA therapy. Time to HTN episode was 0.2 months. There were few instances of major bleeding with either IBR (2%) or ACA (6%). Two pts (4%) developed an invasive infection while on IBR, no evidence of invasive infection has been reported in the pts receiving ACA. At the time of data collection, 3 patients have died, causes of death included sepsis, respiratory failure and multiorgan failure that were not deemed treatment-related.
Conclusion: This real-world experience shows overall good tolerability of ACA after prior IBR therapy in pts with CLL. We observed a lower incidence of Afib (17% vs. 10%) and HTN (10% vs. 2%) during ACA treatment compared to IBR, even if cardiovascular co-morbidities were common among our pts. With a median follow up of 12.4 months (range, 0.4-43.3 months) since the start of ACA therapy, only 27% of pts discontinued treatment because of toxicity.
Disclosures
Thompson:AbbVie, Pharmacyclics, Adaptive Biotechnologies, Genentech: Research Funding; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech, Amgen: Honoraria; AbbVie, Gilead, Janssen, Pharmacyclics, Adaptive Biotechnologies, Genentech: Consultancy. Jain:TransThera Sciences: Research Funding; Ipsen: Honoraria; Newave: Research Funding; Loxo Oncology: Research Funding; Medisix: Research Funding; AbbVie: Consultancy, Honoraria, Other: Travel Support, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Other: Travel Support, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel Support, Research Funding; Incyte Corporation: Research Funding; Kite, a Gilead Company: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding; Fate Therapeutics: Research Funding; Aprea Therapeutics: Research Funding; Genentech, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Takeda: Research Funding; Mingsight: Research Funding; TG Therapeutics: Honoraria; Cellectis: Honoraria, Research Funding; Beigene: Honoraria; MEI Pharma: Honoraria; Dialectic Therapeutics: Research Funding; ADC Therapeutics: Research Funding; BMS: Consultancy, Honoraria, Other: Travel Support, Research Funding; Servier Pharmaceuticals LLC: Research Funding; Precision Biosciences: Consultancy, Honoraria, Other: Travel Support, Research Funding; Pharmacyclics, Inc.: Consultancy, Honoraria, Other: Travel Support, Research Funding; Cellectis: Honoraria, Research Funding; Novalgen: Research Funding; CareDx: Honoraria; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria, Other: Travel Support. Wierda:Sunesis: Research Funding; Pharmacyclics LLC: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Karyopharm: Research Funding; Juno: Research Funding; Bristol Meyers Squibb (Juno and Celgene): Research Funding; Genzyme: Consultancy; Sanofi: Consultancy; Xencor: Research Funding; Kite, a Gilead Company: Research Funding; AbbVie: Research Funding; Janssen: Research Funding; Loxo Oncology, Inc./Lilly: Research Funding; Miragen: Research Funding; AstraZeneca/Acerta Pharma. Inc.: Research Funding; Cyclacel: Research Funding; Genentech: Research Funding; Gilead Sciences: Research Funding; GSK/Novartis: Research Funding. Kantarjian:Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; KAHR Medical Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Research Funding; Novartis: Honoraria, Research Funding; AbbVie: Honoraria, Research Funding; Jazz Pharmaceuticals: Research Funding; Ipsen Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astellas Health: Honoraria, Membership on an entity's Board of Directors or advisory committees; Daiichi-Sankyo: Consultancy, Research Funding; Amgen: Honoraria, Research Funding; NOVA Research: Honoraria; Pfizer: Honoraria, Research Funding; Takeda: Honoraria. Ferrajoli:Beigene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.