Abstract
Background: CLL OS rates have improved over the last 20 years (y), starting with the use of chemotherapy and chemoimmunotherapy (CT/CIT), to the introduction of novel agents such as Ibr. Ibr has shown superiority to standard CT/CIT across a range of 1L pt populations with demonstrated OS improvements in multiple pivotal trials. Recently presented 8-y follow-up data from the first pivotal trial for Ibr demonstrated that more than half of 1L unfit pts remain progression-free. The ideal goal of any therapeutic regimen would be to cure patients of their disease, but the first step could be to provide enough effective therapeutic options to allow patients to live with their disease rather than die from it. Given the size of the program and the length of follow-up, Ibr is uniquely positioned to assess whether the initiation of 1L Ibr could essentially remove the survival hazard associated with CLL vs the general population.
Aims: 1) To compare pooled OS of previously untreated CLL pts treated with Ibr to that of the available age-matched general population 2) To compare the pooled characteristics and OS results with Ibr vs CT/CIT across three phase 3 trials.
Methods: Data were pooled from the RESONATE-2 (NCT01722487), ECOG1912 (NCT02048813), and iLLUMINATE (NCT02264574) clinical studies which evaluated Ibr alone and in combination with other agents in pts with previously untreated CLL/SLL. Individual trial descriptions and key eligibility criteria have been previously published. For the comparison of OS for Ibr-treated pts with that of the available age-matched population, an age-matched approach was applied to pooled Ibr-treated pts to include those who were ≥65 y at the time of initial CLL diagnosis. These pts were then compared to a simulated age-matched OS data cohort from the general population (CDC life table for total US population in 2019). Pooled OS data from Ibr-treated pts from the 3 Ibr studies were compared to OS data from CT/CIT-treated pts. OS was estimated using Kaplan-Meier methodology.
Results: 603 pts with previously untreated CLL/SLL received Ibr treatment across the 3 pooled studies. Among those treated with Ibr, 58.7% received Ibr + rituximab, 22.6% received single agent Ibr, and 18.7% received Ibr + obinutuzumab; the median age was 63 y (range, 31‒89); among pts with available genetic data, 90% (482/538) were without del(17p)/TP53 mutation; median follow-up was 42 months (mo).
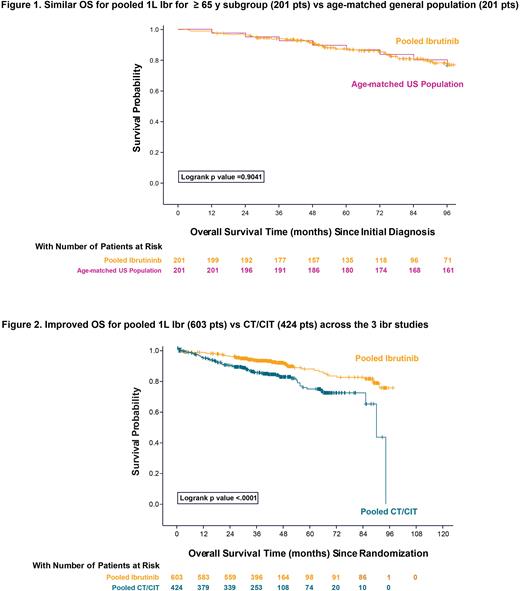
To compare Ibr data to an age-matched general population, 201 pts age ≥65 y at initial diagnosis were pooled from the 3 Ibr studies; median time from initial diagnosis to randomization was 20 mo and the median follow-up from initial diagnosis was 7.6 y. OS estimate at 8-y for Ibr-treated pts aged ≥65 y from time of CLL diagnosis was 78% (95% CI, 71‒84) compared to 77% (95% CI, 70‒82) in the age-matched general population [HR, Ibr over general population, 0.97; 95% CI, 0.63‒1.51; p=0.90]; Figure 1.
Pooled data from the 603 Ibr-treated pts were also compared to data from pts who received CT/CIT (N=424 pts) across the 3 pooled studies. Among pts who received CT/CIT, 41.3% received fludarabine + cyclophosphamide + rituximab; 31.4% received chlorambucil, and 27.4% received chlorambucil + obinutuzumab; the median age was 66.5 y (range, 28‒90); 91% (308/339) of pts with available genetic data were without del(17p)/TP53 mutation; median follow-up was 42 mo. OS estimates were significantly improved with Ibr: 3- and 5- y rates were 93% (95% CI, 91‒95) and 88% (95% CI, 83‒91), respectively, for pts who received Ibr and 85% (95% CI, 81‒89) and 75% (95% CI, 68‒81), respectively, for pts treated with CT/CIT [HR, Ibr over CT/CIT, 0.46; 95% CI, 0.33‒0.66); p<0.0001]; Figure 2.
Conclusion: This pooled analysis suggests that initiating therapy with 1L Ibr improves OS vs traditional CT/CIT regardless of age or fitness. It is also the first demonstration to our knowledge that pts who initiate Ibr over an 8- y follow-up have similar survival estimates as age-matched pts in the general population.
Disclosures
Ghia:Janssen: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; BeiGene: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Lilly/Loxo: Consultancy, Honoraria; MSD: Consultancy, Honoraria; Roche: Consultancy, Honoraria. Owen:Incyte: Honoraria; GIlead: Honoraria; Roche: Honoraria; Merck: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Novartis: Honoraria; AbbVie: Honoraria; BeiGene: Honoraria. Barrientos:Merck, Oncternal, Pharmacyclics/Abbvie: Research Funding; Beigene, AstraZeneca, Pharmacyclics/Abbvie: Consultancy. Barr:Merck, abbive, gilead, Beigene, Genentech, Astrazeneca, Janssen, TG therapeutics, Celgene, BMS, Morphosys, Adaptive: Consultancy. Mato:Octopharma: Honoraria, Research Funding; Pfizer: Research Funding; Janssen: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; DTRM Biopharma: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Genmab: Honoraria, Research Funding; Johnson & Johnson: Honoraria, Research Funding; TG Therapeutics, Inc: Honoraria, Research Funding; LOXO: Honoraria, Research Funding; Nurix: Research Funding; AstraZeneca: Honoraria, Research Funding; Adaptive Biotechnologies: Honoraria; Dava: Honoraria; AbbVie: Honoraria, Research Funding; Curio: Honoraria; Pharmacyclics, LLC: Honoraria, Research Funding; BMS: Honoraria; Medscape: Honoraria; Acerta: Research Funding; PER: Honoraria; PerView: Honoraria. Shi:Everest Clinical Research: Current Employment. Szoke:AbbVie: Current equity holder in private company, Current holder of stock options in a privately-held company; Pharmacyclics, an AbbVie Company: Current Employment. Abbazio:AbbVie: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company; Bristol Myers Squibb: Current equity holder in private company, Current holder of stock options in a privately-held company. Krigsfeld:Bristol Myers Squibb: Other: Travel, Accommodations, Expenses; Inovio: Current equity holder in private company; Dynavax: Current equity holder in private company; Moderna: Current equity holder in private company; Bristol Myers Squibb: Current equity holder in private company; AbbVie: Current equity holder in private company; Pharmacyclics LLC, an AbbVie Company: Current Employment; Bristol Myers Squibb: Ended employment in the past 24 months; AbbVie: Other: Travel, Accommodations, Expenses; Pharmacyclics, an AbbVie Company: Other: Travel, Accommodations, Expenses. Burger:Janssen: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses; Novartis: Honoraria, Other: Travel, Accommodations, Expenses; Pharmacyclics LLC: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Research Funding, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; Gilead: Consultancy, Honoraria, Other: Travel, Accommodations, Expenses, Speakers Bureau; BeiGene: Consultancy, Research Funding, Speakers Bureau; AstraZeneca: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.