Abstract
Background Multiple myeloma (MM) is a plasma cell clonal malignant disease. In the past 20 years, the progression free survival rate and overall survival rate of patients have been significantly improved, however, due to the potential complex cytogenetic abnormalities, the clinical progresses are heterogeneous and the clinical benefits of patients are not consistent, especially for high-risk patients. In Chinese clinical practice, 17p-, t(4;14), t(14;16) are considered high-risk cytological abnormalities for newly diagnosed multiple myeloma (NDMM) patients according to R-ISS stage and 2022 Chinese guidelines for diagnose and treatment for MM.
As a genome guardian, TP53 plays an important role in regulating cell cycle, apoptosis, aging, DNA repair and maintaining gene stability. But the threshold of deletion positive proportion with clinical significance by CD138-sorted FISH was unclear. TP53 mutation is also not regularly examined in Chinese patients. For that aim, our center has taken TP53 seriously as a heterogeneity problem for clinical discussion.
Methods The clinical data of 248 NDMM patients in the First Affiliated Hospital of Soochow University of a VRD clinical study, with VRD in combination with autologous stem cell transplantation or VRD treatment for 8 cycles, from September 1, 2018 to August 31, 2021, were analyzed retrospectively. The samples for CD138-sorted FISH, CytoScan and targeted panel sequencing are obtained from the Multiple Myeloma Specialized Disease Bank of the National Center for Clinical Medical Research of Hematological Diseases.
Results In this study, the deletion rate of TP53 was 13.0% (32/248), the mutation rate was 2.0% (5/248), the incidence of any TP53 event was 14.5% (36/248), and the incidence of double allele event was 0.8% (2/248). The detection rate of TP53 gene mutation was low, which was related to the short clinical application time of targeted panel sequencing with CD138-sorted samples.
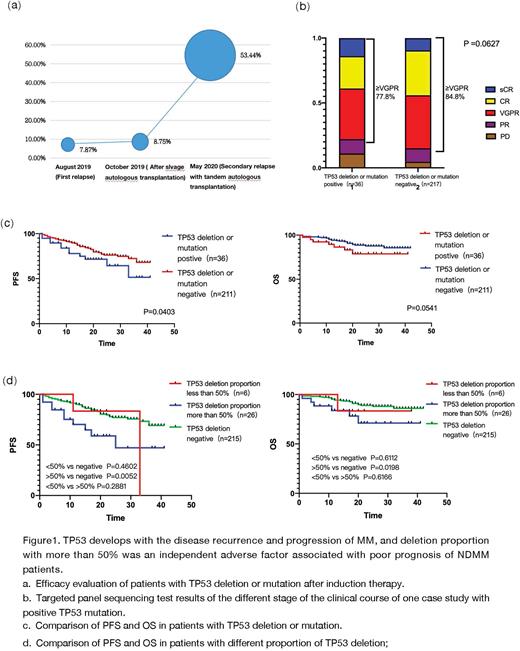
All patients with TP53 deletion or mutation were DS-III stage, with anemia (72.2%), hypoalbuminemia (58.3%) and renal insufficiency (38.9%). The most common accompanied abnormalities were 13q14- (63.9%), followed by t (4;14), 1q21+, t (11;14). Abnormal karyotypes were detected by FISH and CytoScan, indicating gene complexity and instability, and the clonal evolution, which is the root of disease recurrence and progression. The positive rate of TP53 mutation of one case study increased from 7.87% at the first recurrence to 53.44% at the second recurrence with 3rd line therapy (Figure 1a), indicating that TP53 gene lead to disease progression and the formation of multidrug resistance.
In terms of induction effects, patients with TP53 gene abnormalities had ≥VGPR with 77.8%, lower than that of negative group (84.8%, P=0.0627)(Figure 1b). The median follow-up time is 20 months (1-42 months). Compared with negative ones, TP53 positive subgroup had an adverse PFS result (33 months vs not reached, P=0.0403), while the OS difference hasn't been clinical significant (both not reached, P=0.0541) (Figure 1c). The result showed that TP53 deletion with more than 50% was an independent adverse factor associated with poor prognosis of NDMM patients (PFS: HR=2.2441, 95%CI 1.117-5.335, P=0.0025; OS: HR=2.771, 95%CI 1.006-7.629, P=0.049). The prognosis of patients with TP53 deletion more than 50% was significantly worse than that of the negative group (PFS 25months vs not reached, P=0.0032, OS not reached P=0.0198), but TP53 deletion less than 50% had no difference compared with negative group. (Figure 1d).Conclusions Patients with TP53 deletion or mutation have obvious clinical symptoms and are prone to cytogenetic abnormalities such as 1q+, 1p-, -13, IgH translocation, chromosome abnormality and so on. TP53 deletion with more than 50% was an independent adverse factor associated with poor prognosis of NDMM patients, as a result, TP53 deletion more than 50% is the clinical significant threshold for clinical use.
This study comprehensively describes the detection of genetic abnormalities and prognostic survival analysis of NDMM patients by using FISH, CytoScan and targeted panel sequencing. Identifying the real high-risk patients, we can earlier take targeted and more active measures, such as intensive induction treatment, tandem transplantation or CART treatment, to better deepen and consolidate the therapeutic effect for long-term survival benefits of patients.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.