Abstract
The decision to offer Allo-HCT in MDS relies on prognostic scoring models which include clonal cytogenetic (CG) abnormalities, blood counts (cytopenias), and % of marrow blasts (i.e. revised international prognostic scoring system, IPSS-R). Recently, an updated clinical-molecular prognostic model combining genomic profiling with hematologic and CG parameters, namely IPSS-Molecular (IPSS-M), showed better prognostic discrimination across all clinical endpoints and re-stratified almost one-half of patients (pts) vs. IPSS-R. Hence, we evaluated the impact of IPSS-M, CG risk group and individual mutations on Allo-HCT outcomes.
The analysis included 191 consecutive MDS pts undergoing Allo-HCT who had available next generation sequencing (NGS) analysis performed at Mayo Clinic (at diagnosis (Dx, n=164 or prior to Allo-HCT, n=162) between 11/2015 and 1/2022. Median age at Allo-HCT was 64 (18-75) yrs, and 112 (58.6%) were male. At Dx, 126 (77%) pts had at least one pathogenic genetic alteration, including (>5% incidence) ASXL1 (28.0%), TP53 (18.9%), TET2 (14.6%), SRSF2(12.2%), U2FA1 (12.2%), DNMT3A (8.5%), BCOR (7.9%), RUNX1 (6.7%), and SF3B1 5.5%; a similar mutational pattern was present at Allo-HCT: ASXL1 (28%),TET2 (15.2%) U2AF1 (13.4%), TP53 (12.2%), SRSF2 (11%), RUNX1(9.8%), DNMT3A (7.3%), SETBP1 (7.3%), SF3B1 (6.7%), BCOR (6.7%), STAG2 (5.5%), and JAK2 (5.5%).
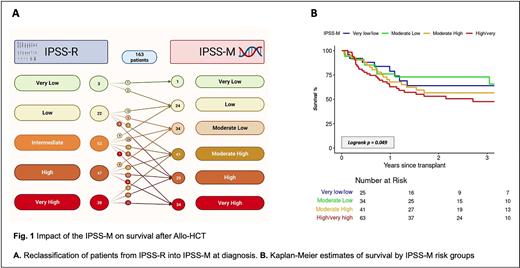
Most pts (156, 81.7%) received at least 1 line of therapy, and 45 (23.6%) had >5% marrow blasts at Allo-HCT. The majority (134, 70%) received reduced intensity conditioning. Graft versus host disease (GVHD) prophylaxis consisted of methotrexate/tacrolimus in 138 (77.5%) and post-transplant cyclophosphamide in 40 (22.5%). HCT comorbidity index was >3 in 113 (59%). IPSS-M risk group was calculated and reclassified from IPSS-R into very low (1, 0.6%), low (24, 14.7%), moderate low (34, 20.9%), moderate high (41, 25.2%), high (29, 17.8%), very high (34, 20.9%), mainly for IPSS-R intermediate and high-risk groups (Fig. 1).
Median F/U after Allo-HCT was 18.8 (0.4-76.8) months. The 100-day and 1-year non-relapse mortality (NRM) were 7.9% and 18.1%, respectively. The 2-year incidence of death and relapse were 40.1% (95% confidence intervals (CI) 33.5-48%) and 21.6% (95%CI 16.4-28.4%), respectively. Cumulative incidences of grade III-IV acute GVHD was 10% and 1-year cumulative incidence of extensive chronic GVHD was 12%.
In multivariable analysis, IPSS-M predicted mortality [Hazard ratio (HR) (per 1 risk category) 1.21, 95%CI 1.00-1.47, p=0.049] and relapse (HR=1.47, 95%CI 1.13-1.92, p=0.004), but not NRM (HR 0.95, 95%CI 0.74-1.22). The 1-year mortality rates for very low/low, moderate-low, moderate-high, high/very high risks were 21%, 24%, 32%, and 37%, respectively (Fig. 1).
Individual mutations had minimal independent association with outcome in the multivariable model. For mortality, only ASXL1 at Dx (HR 0.48, 95%CI 0.26-0.91, p=0.024) and SF3B1 at Allo-HCT (HR 2.51, 95%CI= 1.06-5.96, p=0.037) were significant. TP53 at Dx had a borderline association (HR 0.46, 95% CI= 0.942.69, p=0.086). Also, there was no significant association of individual mutation with relapse (including TP53 at Allo-HCT, HR 1.77, p=0.085). Only SF3B1 at Allo-HCT correlated with NRM (HR 3.16, 95%CI= 1.22-8.17, p=0.018). Conversely, CG risk group remained strongly associated with mortality (p=0.007) and relapse (p=0.003), particularly poor/very poor (P/VP) risk CG (Mortality: HR 2.46, 95%CI 1.44-4.19, p=0.001; Relapse: HR 3.46, 95%CI 1.70-7.05, p=0.0006). There was no association of CG risk with NRM.
We demonstrate value of IPSS-M risk stratification in predicting outcomes after Allo-HCT for MDS, re-stratifying pts into more discrete prognostic groups that predict mortality and relapse risk. Hence, IPSS-M could inform post allo-HCT decision including future maintenance strategies to prevent relapse in high/very high IPSS-M. CG risk group (esp. P/VP risk), a core component of IPSS-M, independently contributes to outcome prediction both within the model and individually. Disease risk does not predict NRM, and individual mutations have limited association with outcome, suggesting allo-HCT may mitigate the poor prognostic impact of high-risk oncogenic mutations present at Dx, including TP53 and ASXL1. An updated analysis on mutational complexity will be shown at the meeting.
Disclosures
Khera:Incyte: Consultancy; Optum: Honoraria. Litzow:Abbvie: Research Funding; Amgen: Research Funding; Astellas: Research Funding; Novartis: Research Funding; Syndax: Research Funding; Jazz: Consultancy; Actinium: Research Funding; Pluristem: Research Funding; Biosight: Other: Data Monitoring Board. Mangaonkar:Bristol Myers Squibb: Research Funding. Palmer:PharmaEssentia: Consultancy, Research Funding; Protagonist: Consultancy; Sierra Oncology: Consultancy; CTI BioPharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Shah:Astellas: Research Funding; Celgene: Research Funding; Marker Therapeutics: Research Funding. Foran:AbbVie, Actinium, Aptose, Astex, H3Biosciences, Kura Oncology, Trillium, Xencor: Research Funding; Novartis, Servier, Pfizer, BMS, Taiho: Other: Formal Advisory Activities.
Author notes
Asterisk with author names denotes non-ASH members.