Abstract
Background: Patients with transfusion-dependent β-thalassemia (TDT) require life-long RBC transfusions accompanied by iron chelation. Prepubertal children account for the vast majority of TDT patients in China. Re-activation of fetal γ-globin gene expression by suppression of BCL11A has been shown to be one potential cure for TDT. Early data shown that editing of distal CCAAT in the γ-globin gene (HBG1 and HBG2) promoters by CRISPR-Cas9 can also re-activate γ-globin expression, suggesting a possiblility to cure TDT through directly editing of γ-globin gene.
Aims: We initiated the study to explore the safety and efficacy of autologous HBG1/2 promoter-modified CD34+ hematopoietic stem and progenitor cells (RM-001) in TDT (ChiCTR2100053406). RM-001 was produced through CRISPR-Cas9 ribonucleoprotein (RNP)-mediated editing of the patient's autologous CD34+ (HSPCs), disrupting CCAAT box in the HBG1 and HBG2 promoters to mimick naturally occurring mutations that lead to hereditary persistence of fetal hemoglobin (HFPH) . Here, we present preliminary safety and efficacy results of the first two TDT patients less than 9 years old that treated with RM-001.
Methods: Patients (6-35 y of age) with TDT receiving packed red blood cell (pRBC) transfusions of ≥100 mL/kg/y or ≥10 units/y in the previous 2 y were eligible. Peripheral CD34+ HSPCs were collected by apheresis after mobilization with G-CSF and plerixafor. CD34+ cells were edited with CRISPR-Cas9 using a guide RNA specific for the distal CCAAT box on the HBG1/2 promoter. Prior to RM-001 infusion, patients received myeloablative conditioning with busulfan via CVC infusion for 4 consecutive days form day-7 to day-3, at a starting dose 1.0 mg/kg as a 2-hour infusion every 6 hours for a total of 16 doses. Patients were monitored for stem cell engraftment/hematopoietic recovery, adverse events (AEs), Hb production, HbF and F-cell expression, pRBC transfusion requirements.
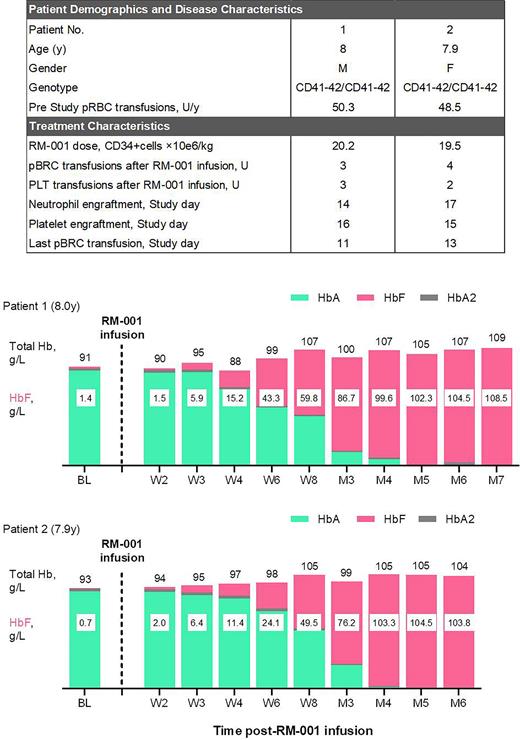
Results: Data presented here for the two patients of 8.0/7.9 year old at the time of RM-001 infusion, both were β0/β0 [CD41-41/CD41-42] genotype, with an annualized packed red blood cell (pRBC) transfusion of 50.3 and 48.5 units/y, respectively (Figure). Patients received a single dose of RM-001 cells with CD34+ cells dose of 20.2x106/kg and 19.5x106/kg. Patients achieved neutrophil engraftment at Day14 and Day17 after RM-001 infusion respectively. Patients achieved platelet engraftment very fast (Day16 and Day15) and received total amount of 3 and 2 units platelet infusion respectively. Patients ceased pRBC transfusions within 1 month after RM-001 infusion and received total amount of 3 and 4 units pRBC respectively. HbF level increased over time in both patients after RM-001 infusion, reached ~100 g/L at 4 month post-RM-001 infusion (Figure). No serious adverse event (SAE) occurred. The safety profile was generally consistent with busulfan myeloablation and autologous hematopoietic stem cell transplantation.
Summary/Conclusion: In this study, two prepubertal children with TDT were treated successfully with autologous HSPCs of which HBG1/2 promoters were modified by CRISPR-Cas9 system. Both patients treated with RM-001 demonstrated rapid and successful engraftment, with much less pRBC and platelet requirement compared with allogeneic HSCT. Moreover, both patients achieved transfusion-independent within 2 month after RM-001 infusion owe to the rapaid increase in HbF levels. No RM-001 related or possibly related SAE report. This initial result indicates that RM-001 is a promising treatment for TDT patients.
Data will be updated for the presentation.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.