Abstract
Introduction Peripheral T-cell lymphomas (PTCL) are rare, heterogeneous, aggressive non-Hodgkin lymphomas associated with high rates of relapse and guarded prognosis. Currently, treatment regimens for PTCL vary across institutions with respect to both selection of induction therapy, and inclusion of consolidative high-dose chemotherapy and ASCT (autologous stem cell transplantation). Safety-net hospitals often face limitations in treatment options like cellular therapies due to patient access to care, insurance barriers, and treatment funding. This study analyzed the demographics, treatment regimens, and survival outcomes among PTCL patients treated at a public safety-net hospital versus a private tertiary academic institution within the same healthcare system. The primary aim of the study was to analyze the utilization and impact of consolidative ASCT on survival outcomes in PTCL patients.
Methods We conducted an IRB approved, retrospective review of 108 patients diagnosed with PTCL who were treated within our healthcare system between January 2009 and December 2020. The main variables studied include comparison of demographics, PTCL histologic subtype, disease stage, Prognostic Index for T-cell lymphoma (PIT) score, chemo-based induction modalities, and survival outcomes. Hypothesis testing was performed using chi-square tests, t-tests, and other nonparametric tests as required, with a 2-tailed P value ≤ .05. Survival analysis for progression-free (PFS) and overall survival (OS) was performed with the Kaplan-Meier methodology and evaluated with the log rank test. Univariate and multivariate hazard regression was used to assess the relationship between pre-specified variables and the primary endpoint of OS, and secondary endpoints of PFS and time to next treatment (TTNT).
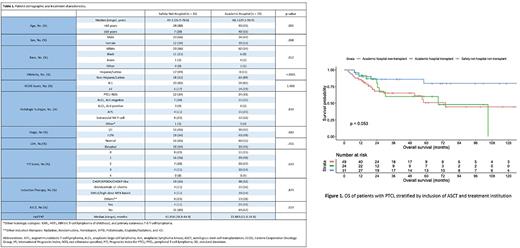
Results Among the 108 patients with PTCL, 35 (32%) were treated at a safety-net hospital, whereas 73 (64%) were treated at a tertiary academic center. In comparison with those treated at the academic institution, a significantly higher proportion of patients treated at the safety-net hospital were younger than 65 years (P = .002), African American (P = .005), or Hispanic/Latino (P < .0001). When comparing the two patient cohorts, there were no significant baseline differences in the distribution of PTCL subtypes, patient prognostic factors, or induction modality. Overall, 28 (26%) patients underwent consolidative ASCT, 21 (75%) in first complete remission (CR1). Notably, there were no significant differences in OS and PFS by the treating institution (P = .148 and P = .173, respectively) or ASCT status (P = .114 and P = .264, respectively). On multivariate analysis, ASCT in CR1 was independently associated with inferior OS (HR 5.27; 95% CI, 1.78 to 15.58; P = .003). There was no association between ASCT in CR1 and PFS (HR 1.63; 95% CI, 0.74 to 3.57; P = .22) or TTNT (HR 0.63; 95% CI, 0.26 to 1.53; P = .30). Age at diagnosis and response to initial therapy were significantly associated with OS, PFS and TTNT (all P < 0.05).
Conclusions In our analysis, patients with PTCL who received ASCT consolidation demonstrated worse survival outcomes, even after adjusting for several cofounding factors. These findings are likely multifactorial with disease and patient heterogeneity as well as multidisciplinary standardized treatment practices at both sites likely contributing to improved safety-net hospital outcomes. This study is limited by its small sample size and retrospective design. Research into the utility of consolidative ASCT in PTCL has the potential to personalize treatment paradigms for this unique set of patients.
Disclosures
Anderson:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kaur:UT Southwestern: Consultancy, Current Employment. Madanat:Sierra Oncology, Stemline Therapeutics and Novartis: Membership on an entity's Board of Directors or advisory committees; BluePrint Medicines, GERON, OncLiv: Consultancy, Honoraria. Awan:Genentech: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; Janssen: Consultancy; Pharmacyclics: Consultancy, Research Funding; Gilead Sciences: Consultancy; Kite Pharma: Consultancy; Celgene: Consultancy; Karyopharm: Consultancy; MEI Pharma: Consultancy; Verastem: Consultancy; Incyte: Consultancy; BeiGene: Consultancy; Johnson and Johnson: Consultancy; Dava Oncology: Consultancy; BMS: Consultancy; Merck: Consultancy; Cardinal Health: Consultancy; ADCT Therapeutics: Consultancy; Epizyme: Consultancy; Caribou Biosciences: Consultancy; Cellecter Bisosciences: Consultancy. Ramakrishnan Geethakumari:Kite: Consultancy; BMS: Consultancy; Rafael Pharma: Consultancy; Pharmacyclics LLC: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Cellectar Biosciences: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.