Abstract
Background: Erythropoietic protoporphyria (EPP) caused by FECH and ALAS2 mutations results in toxic accumulation of photoreactive protoporphyrin IX (PPIX). High levels of PPIX result in excruciating phototoxic reactions and hepatopathy caused by biliary stasis. Reduction of PPIX is associated with amelioration of disease in the settings of hematopoietic stem cell transplant, pregnancy and extracorporeal photoinactivation.
Bitopertin is a small molecule inhibitor of glycine transporter 1 (GlyT1), which imports large amounts of extracellular glycine into erythropoietic precursors. GlyT1 is needed to supply adequate amounts of substrate for the heme synthesis pathway to enable the large amounts of hemoglobin needed for normal red blood cell production. Treatment of EPP mouse models with FECH and ALAS2 mutations resulted in 45-73% reduction in PPIX, as compared to controls (Hong et al. 2021). These data, combined with a well-defined safety profile that has previously been established in over 4,000 patients and healthy volunteers motivates the current study to evaluate this potentially disease-modifying treatment.
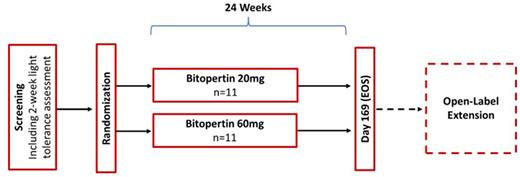
Study Design and Methods: This is a Phase 2, randomized, open-label, parallel arm trial of 20 and 60 mg bitopertin daily for 24 weeks in EPP patients. The trial is being conducted at 2 sites in Australia. The primary endpoint is percent change in metal-free PPIX. Secondary endpoints include patient reported outcomes of light tolerance, safety and tolerability, and PK parameters. Eligibility criteria include: age ≥18 years with EPP by FECH/ALAS2 genotyping or porphyrin analysis, AST/ALT <2x upper limit of normal, normal bilirubin, and hemoglobin ≥10 g/dL. Up to 22 participants will be randomized and stratified by baseline daily light tolerance (< or ≥ 30 minutes) and study site. Study sample size calculations were not performed for this first in EPP study. Endpoints will be summarized using descriptive statistics.
Results: Subject enrollment began July 2022. Initial data will be presented at the meeting.
Disclosures
Mensing:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Howell:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. Chan:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company. MacDonald:Disc Medicine: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company. Savage:Disc Medicine: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.