Abstract
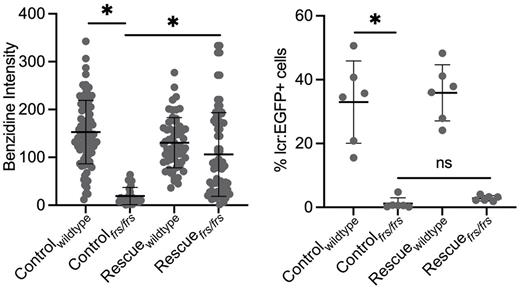
Developing erythroid cells are the main consumer of iron in vertebrates, as their main function is to oxygenate tissues via hemoglobin. Iron metabolism is a key driver of erythroid development and hemoglobin synthesis. When iron deficient, or when iron metabolism is dysregulated, erythroid cells are both lacking in hemoglobin and exhibit differentiation defects. Currently, the efficacy of iron supplementation in patients is monitored by quantitating hemoglobin levels. However, the effect of iron supplementation on erythroid differentiation is less clear. As hemoglobinization and erythroid differentiation are inextricably intertwined with erythroid function, we sought to understand whether and how iron supplementation functions to restore erythroid differentiation during iron deficiency. Tackling this problem in vivo has been fraught with technical challenges as vertebrates with mutations in iron metabolism genes often die from anemia in utero. To circumvent this problem, we used zebrafish with genetic iron metabolism defects to determine if iron supplementation could rescue erythropoietic defects at both the cellular and systemic level. Zebrafish are tractable in vivo models for such studies as they develop externally and can easily be treated by exogenous iron in their water. To carry out this study, we designed a system to sort Tg(globin lcr:eGFP) erythrocytes from single zebrafish embryos onto slides for imaging, while keeping sufficient numbers of cells for genotyping of the same embryo. We used two zebrafish lines which carry loss of function mutations in the mfrn1 (carrying a mitochondrial iron trafficking defect) and fpn1 (carrying a defect in iron export from intestinal and yolk syncytial epithelium-a model of dietary iron deficiency). Both lines had decreased erythroid cell numbers. In addition, the erythroid cells were hemoglobin deficient and had erythroid differentiation defects which included enlarge nuclei. We showed that exogenous iron supplementation (labeled as "rescue) in mfrn1 mutant zebrafish restored hemoglobinization (benzidine staining) but not erythroid cell number (% lcr:eGFP) or terminal differentiation. However, iron supplementation in fpn1 mutant zebrafish, restored erythroid cell number but not terminal differentiation defects. The main difference in the 2 mutants is that the mfrn1 mutant has a defect in intracellular iron trafficking leading to defective iron metabolism, while the fpn1 mutant has defective iron export to erythroid cells but their erythroid cells have intact intracellular iron trafficking. mfrn1 mutants had defects in cell cycle progression and increased apoptosis in their erythroid cells which likely is a cause for decreased erythroid cell numbers. Our data suggests that in addition to adequate iron levels, correct regulation of iron trafficking is required for optimal erythroid iron utilization, terminal erythropoiesis and potentially, proliferation and differentiation of erythroid progenitors. This may indicate a role for iron transporters in directing iron fate, as well as signaling to developmental pathways.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.