Abstract
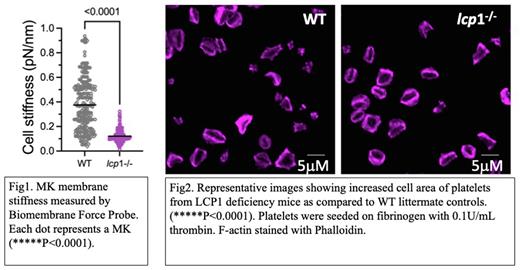
An adequate number of normally functioning platelets is required for normal hemostasis, while thrombocytopenia and platelet dysfunction are common causes of pathologic bleeding. Platelets are released into the blood from proplatelets (PPs) that extend from megakaryocyte (MK) intracellular invaginated membrane system (IMS) that is continuous with the plasma membrane. Tubulin provides the motor for PP extension, and actin reorganization is believed to be critical for MK migration and PP branching. However, the role for actin in PP formation (PPF) is less well understood. The initiation of PPF requires podosomes - actin-based structures with an F-actin core surrounded by a ring of adhesion molecules (e.g., integrins) and many actin-binding proteins. We have been intrigued by how podosomes form and how the IMS is able to penetrate the dense cortical actin cytoskeleton to form PPs. We recently showed that human MKs and platelets express a novel actin bundling protein, L-plastin. L-plastin levels were inversely correlated with platelet number, and its expression was down-regulated during MK maturation. L-plastin knockdown in cultured human CD34+ derived MKs caused increased PP, IMS, and podosome formation. These data indicate L-plastin is a negative regulator of MK platelet production. To assess the in vivo effects of L-plastin, we have investigated platelet production and function in vivo using L-plastin knockout (KO) mice (Lcp1-/-). Compared to wildtype (WT) littermate controls, Lcp1 KO mice showed both significantly increased platelet counts and increased PPF from cultured murine bone marrow MKs. Elegant work in both HUVECs and epithelial cells has shown high membrane proximal F-actin (MPA) prohibits membrane protrusions, and lowering MPA density is required to sensitize areas for membrane protrusion (Bisaria, Science 2020). We hypothesize that normal MK levels of L-plastin inhibits PPF in less mature MKs by F-actin bundling, which raises the MPA. Conversely, lower L-plastin levels result in lower MPA and enabling PP protrusion. Enhanced L-plastin-mediated F-actin bundling is expected to increase membrane stiffness, so we measured MK membrane stiffness at single cell level using a state-of-the-art Biomembrane Force Probe (BFP). MKs from KO mice showed 68% reduction in membrane stiffness as compared to WT controls (p<0.0001) (Fig 1). Ultrastructure of MKs also revealed increased IMS in Lcp1-/- MKs. Preliminary data suggests that L-plastin deficiency also associated with increased MK podosome formation in vitro, which is consistent with our findings in human CD34+ derived MKs. Although L-plastin levels decrease during terminal MK differentiation, they persist at moderate levels in platelets. Platelet integrin activation, granule release and clot retraction also depend on actin reorganization, but L-plastin function in platelets has not been studied. We observed no difference between WT and Lcp1 KO platelets for measures of inside-out integrin signaling (activation-induced JON/A binding or P-selectin expression). However, when plated on fibrinogen and stimulated with thrombin, platelets from KO mice showed significantly increased spreading compared to WT mice (Fig 2). A similar trend was observed for platelet clot retraction. Taken together these results show that L-plastin regulates MK membrane stiffness, which likely inhibits PPF. L-plastin also appears to participate in platelet outside-in, but not inside-out, integrin signaling. On-going studies are determining whether integrin beta3 is the molecular link between these two findings.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.