Abstract
NUP98-rearrangements (NUP98r) are the second most common driver gene alteration in relapsed pediatric acute myeloid leukemia (AML) and are associated with poor prognosis. There are over 30 different fusion partners, and some partners are associated with specific disease phenotypes. For example, NUP98::NSD1 is commonly found in AML with myelomonocytic differentiation, whereas NUP98::KDM5A is predominantly found in acute megakaryoblastic leukemia and occasionally in acute erythroid leukemias. Although rare cellular models of NUP98::KDM5A have been described (Blood. 1990 Mar 15;75(6):1252-61, Blood Adv. 2019 Nov 12;3(21):3307-3321), there is a lack of appropriate models of other NUP98 fusions to investigate how different fusions contribute to leukemogenesis and disease phenotypes. Here we established various human NUP98r leukemia models using cord blood CD34+ cells (CB CD34) and characterized their molecular features.
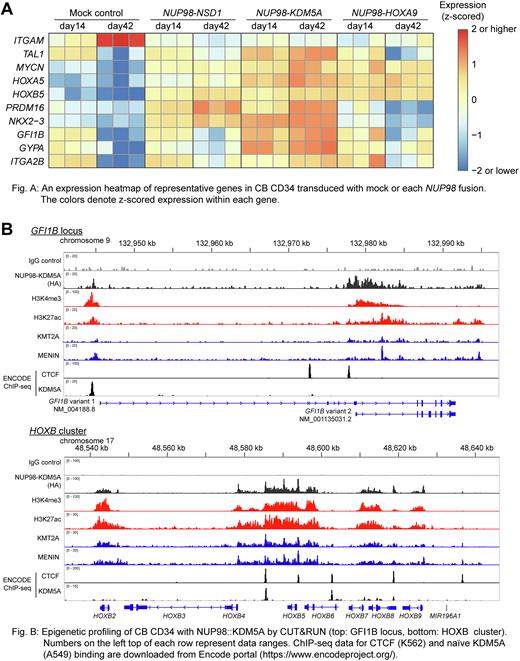
We first transduced CB CD34 with lentiviral particles encoding cDNAs for NUP98 fusions found in AML patients (NUP98::NSD1, ::KDM5A, ::HOXA9, ::BPTF, and ::ZFX), all of which enhanced cell growth in liquid culture and increased clonogenic potential in methylcellulose. We then focused on three major fusions (NSD1, KDM5A, and HOXA9) and investigated transcriptional profiles using RNA sequencing (Fig A). NUP98r-transduced conditions showed high TAL1 and MYCN in addition to upregulation of HOXA and HOXB cluster genes, NKX2-3, and PRDM16, which are features of NUP98r AML in patients, whereas mock control conditions showed rapid downregulation of TAL1 and upregulation of ITGAM (encoding CD11b) upon differentiation. Notably, the expression of genes involved in erythromegakaryocytic differentiation, such as GFI1B, ITGA2B (encoding CD41a), and GYPA (encoding CD235a), are uniquely high in cells with NUP98::KDM5A, indicating that the erythromegakaryocytic phenotypes of NUP98::KDM5A AML are fusion-intrinsic.
To further study how NUP98 fusions drive leukemogenesis, we profiled the genomic binding of fusion proteins (N-terminal HA-tagged NUP98::NSD1, ::KDM5A, ::HOXA9) using anti-HA antibody together with histone modifications (H3K4me3 and H3K27ac) and MLL complex binding (KMT2A and MENIN) using CUT&RUN technology. These profiling studies revealed significant overlaps between direct targets of each NUP98 fusion protein (p-values < 10-13 in all combinations), with a core group of 90 genes bound by all three NUP98 fusion proteins that includes genes with well-known functions in leukemogenesis and hematopoiesis (e.g., CDK6, HOXB3, MECOM). However, each fusion protein also showed specific target genes (KDM5A: 5806 genes, HOXA9: 693 genes, NSD1: 90 genes), and notably, NUP98-KDM5A uniquely bound to an alternative promoter of GFI1B (Fig B), indicating that direct regulation of GFI1B may be a driver of erythromegakaryocytic phenotypes of NUP98::KDM5A AML.
Detailed examination of epigenetic data of CB CD34 with NUP98::KDM5A found that NUP98::KDM5A preferentially bound to upregulated genes over mock controls as well as significant overlaps with positive histone marks (H3K4me3 and H3K27ac) and the MLL complex, in accordance with a recent report that MLL complex is critical for the epigenetic status of NUP98 leukemia (Blood. 2022 Feb 10;139(6):894-906). NUP98::KDM5A bound to the HOXA and HOXB cluster broadly over CTCF binding sites and boundaries of topology-associated domains (TADs), accompanied by super-enhancers marked by H3K27ac (Fig B). Integration of ChIP-seq data of endogenous KDM5A binding from ENCODE project (Nature. 2012 Sep 6;489(7414):57-74) revealed a significant overlap between NUP98::KDM5A targets and endogenous KDM5A binding (p = 7.5*10-105). However, the remaining 4792 genes with peaks (82.5%) were unique to NUP98-KDM5A fusion protein, suggesting interplay between the N-terminus of NUP98 and the C-terminus of KDM5A may create novel DNA binding sites, including the alternative promoter of GFI1B.
In conclusion, our in vitro human models of NUP98r leukemia recapitulate biological features of AML in patients, including growth advantage, clonogenic potential, and transcriptional signatures. Further, these models enable us to investigate the epigenetic profiles unique to each NUP98 fusion, which may provide insight into the biology of this heterogeneous and refractory molecular subtype of pediatric AML.
Disclosures
Miller:Janssen: Pharmaceutical Companies of Johnson & Johnson: Current Employment. Mullighan:FAZE: Honoraria; Consulting: Honoraria; Illumina: Honoraria; Pfizer: Research Funding; Abbvie: Research Funding; BEAM: Honoraria; Amgen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.