Abstract
Background: We have previously shown that bone marrow-specific loss of an adapter protein and a negative regulator of Abelson (ABL) kinase - Abelson Interactor 1 (ABI1), induces myeloproliferative neoplasm-like phenotype in mice resembling human primary myelofibrosis (PMF). This phenotype was mechanistically linked to upregulated NF-κB/STAT3/SRC Family Kinase (SFK) signaling axis. Loss of ABI1 transcript was also noted in human PMF CD34+ stem/progenitor cells and granulocytes (Chorzalska et al., Blood 2018). Primarily due to significant upregulation of NF-κB signaling, ABI1 deficient hematopoietic stem cells (HSC) showed elevated proliferative potential and propensity to exhaustion as reflected in their incapacity to successfully repopulate sublethaly irradiated recipients, arguing against the clonality of the ABI1-loss induced disease. To verify that loss of ABI1 is clonal and results in transplantable disease we performed series of bone marrow transplantations (BMT) using whole bone marrow isolated from both young (10 weeks) and old (52 weeks-old) ABI1-deficient donors. To further confirm clonality and HSC dependence of the phenotype of ABI1 KO MPN model, we used a low dose irradiation (LDI) as a therapeutic modality. LDI is known to be highly toxic to HSC specifically (e.g., Rodrigues-Moreira et al., Cell 2017). We compared LDI therapeutic potential to NF-κB modulator Bortezomib, SFK inhibitor - Dasatinib and JAK2 inhibitor - Ruxolitinib.
Methods: Whole bone marrow was isolated from young (8-12 weeks-old) and old (52-54 weeks-old) ABI1 WT or ABI1 KO CD45.1+ donor mice (Abi1(fl/fl);Mx1-cre(-) or Abi1(fl/fl);Mx1-cre(+) respectively, Chorzalska et al., Blood 2018) and 10x106 cells were transplanted into 1100cGy myeloablated CD45.2+ recipient mice (n=10 mice per genotype per age group). Chimerism of primary transplant and donor-derived populations of granulocytes, T cells, B cells, erythroid cells and macrophage/monocytes were assayed by flow cytometry monthly for 6 months. At this time point, BMT recipients were euthanized and disease progression was determined by gross pathology and histology. Secondary BMTs were performed at this time. In addition, a cohort of 40 ABI1 KO mice were aged for 35-38 weeks (time of disease manifestation in ABI1 KO mice) and divided into 5 groups (n=8, 4 females, 4 males): untreated, subjected to single dose of 100 cGy LDI, implanted with osmotic ALZET pumps with Bortezomib (0.15 mg/kg/day/28days), Ruxolitinib (60 mg/kg/day/28days) or Dasatinib (10 mg/kg/day/28days). Drug treatments were ongoing for 28 days and at this point the animals were euthanized and disease progression was determined by flow cytometry, gross pathology and histology.
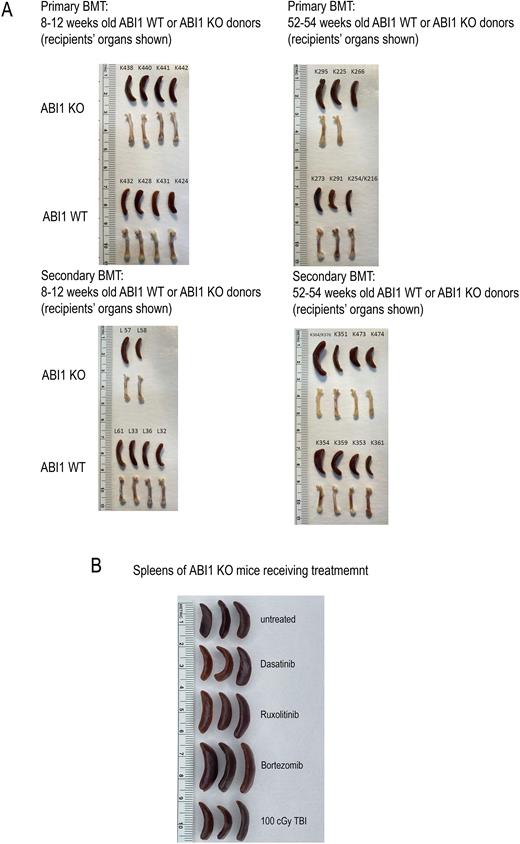
Results: Both primary and secondary transplant performed using bone marrow isolated from young ABI1 WT or ABI1 KO donors showed evidence of progressive MPN with granulocytosis, monocytosis, lymphopenia, erytropenia, 2-grade fibrosis in femurs and spleens, femur pallor and splenomegaly (Panel A). Evidence of more aggressive disease, as determined by increase in spleen size and increased number of immature myeloid cells in the bone marrow, was noted in animals from secondary transplant injected with bone marrow isolated from 52-54-week-old donor ABI1 KO mice. Animals treated with Ruxolitinib, Dasatinib and Bortezomib for the duration of 28 days using ALZET osmotic pumps have not showed significant signs of disease remission as assessed by flow cytometry and evident by spleen sizes. Interestingly, animals which received single dose of 100 cGy, post-28 days showed significant signs of disease remission as observed by decrease in spleen sizes as well as decrease in frequencies of granulocytes and monocytes and normalization in frequencies of T- and B-cells (Panel B).
Conclusions: Our data indicate that loss of ABI1 results in malignant transformation of HSC leading to the development of clonal MPN disease as shown in serial transplantation experiments. Our data also show that low dose irradiation exhibits a curative potential in ABI1-loss driven model of MPN in contrast to 28-day treatment with Bortezomib, Ruxolitinib or Dasatinib. In sum, our data indicate that HSC-driven ABI1-induced clonal disease can be effectively suppressed with low dose irradiation. Testing of LDI as a new therapeutic modality in other models of MPN is warranted.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.