Abstract
Background: Measurable residual disease (MRD) is an important biomarker in acute myeloid leukemia (AML). Among patients who achieve remission after standard chemotherapy, detection of MRD (MRD+) after two cycles of intensive chemotherapy, at the end of consolidation and/or before allogeneic stem cell transplantation (alloHSCT) is a strong prognostic factor for relapse and shorter overall survival (OS) (Short NJ, JAMA Oncol. 2020). Optimal MRD assessment time-points, methodologies, and cut-offs, as well as whether elimination of MRD with subsequent chemotherapy improves outcomes, remain open questions. PALG-AML1/2016 study aims to compare the safety and efficacy of two commonly used induction and salvage regimens in AML (NCT03257241) and is the first international randomized trial in AML induction to prospectively evaluate the impact of MRD on overall survival using multi-modality testing (flow-cytometry, FC; next-generation sequencing, NGS) of serial samples.
Study Design: In this ongoing study, 582 adult patients with newly-diagnosed AML are randomized to daunorubicin and cytarabine +/- cladribine induction. Patients with complete remission (CR) or CR with incomplete hematologic recovery (CRi) or CR with partial hematologic recovery (CRh) receive further post-remission therapy based on predefined risk groups. Serial samples for MRD are collected at time of CR/CRi/CRh after one or two induction cycles (MRD1), and after each consolidation cycle (MRD2, -3, -4).
Material and Methods. The aim of this preliminary analysis was to evaluate the kinetics of MRD using multiparameter flow cytometry (MFC) during post-remission treatment and the influence of subsequent consolidation cycles on MRD status. MRD was assessed according to ELN recommendations using the threshold of ≥0.1% for MRD positivity (MRD+).
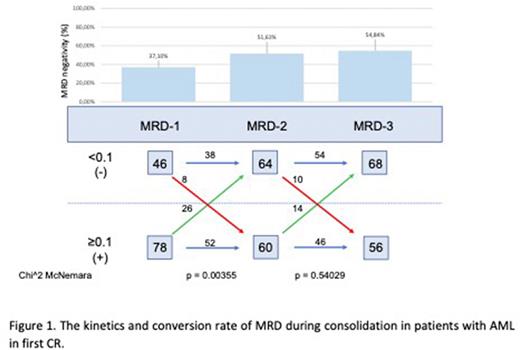
Results. To date of analysis, 408 pts have been enrolled (female 54.9%), 366 (89.7%) with de novo AML and 42 (10.3%) with secondary AML, with mean age 45 years. Responses are evaluable in 333 pts. CR, CRi, CRh after single or double induction were achieved in 211 (63.4%), 55 (16.5%) and 2(0.6%) patients, respectively leading to an overall composite CR (cCR) rate of 80.5%. Forty-eight (14.4%) pts were non-responders and 17 (5%) pts died in aplasia. To date, 124 pts with cCR, have data available on consecutive MRD1, 2 and 3 time points. Among these pts, 118 (95%) pts achieved cCR after single induction and 6 (5%) after double induction. MRD negative (MRD-) cCR was observed in 46/124 (37.1%) pts. Overall, baseline characteristics were generally similar between MRD+ and MRD- pts regarding median age (45 vs. 48 years), WBC at diagnosis (5.8 vs. 11.0 G/L) as well as de novo vs. sAML (89.1% and 10.9% vs. 90.7% and 9.3% respectively). There was a trend toward higher frequency of poor-risk ELN category among MRD+ pts (42.2% vs. 30.1% respectively; p=0.066).
After 1st consolidation (MRD2), the conversion rate from from MRD+ to MRD- was 33.3 % (n=26); MRD- to MRD+ conversion was also noted in 17.4% (n=8), ultimately resulting in a significantly increased rate of MRD- cCR rate to 51.1% (p=0.0035). After second consolidation (MRD2), MRD+ to MRD- conversion was 23.3 % (n=26) and MRD- to MRD+ was 15.6% (n=10), leading to an insignificant further increase in MRD- cCR to 54.8% (p=0.54).
Conclusions: Preliminary data from PALG-AML1/2016 suggest that post-remission consolidation with a single cycle of intensive chemotherapy results in improved rates of MRD negativity, but the incremental value of additional consolidation cycles is uncertain. Loss of MRD negativity is observed in a proportion of patients even at early post-remission time points. Data correlating MRD status and relapse-free survival (RFS) will be presented at the meeting.
Disclosures
Wierzbowska:Gilead: Honoraria; Swixx Biopharma: Honoraria, Research Funding; Astellas: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Celgene/BMS: Honoraria; Novartis: Honoraria; Servier: Honoraria. Pluta:Angelini: Honoraria; Swixx Biopharma: Honoraria, Research Funding; Novartis: Honoraria; Celgen/BMS: Honoraria; Astellas: Honoraria. Czyz:Takeda: Honoraria; Amgen: Honoraria; Pfizer: Honoraria. Libura:Novartis: Honoraria; Servier: Honoraria. Stelmach:Novartis: Honoraria. Czemerska:Sandoz: Honoraria; Celgene: Honoraria; Pfizer: Honoraria; Novartis: Honoraria; Abbvie: Honoraria. Helbig:Novartis: Honoraria. Sobas:Novartis: Honoraria; Celgene/BMS: Honoraria. Wróbel:Novartis: Honoraria; Roche: Honoraria, Research Funding; Takeda: Honoraria; Amgen: Honoraria, Research Funding; Janssen: Honoraria; Beigene: Honoraria; Celgen/BMS: Honoraria; Gilead: Honoraria; Abbvie: Honoraria; GSK: Honoraria. Gil:Gilead: Honoraria; Astellas: Honoraria; Abbvie: Honoraria; Celgene/BMS: Honoraria; Novartis: Honoraria; Pfizer: Honoraria; Janssen: Honoraria. Bieniaszewska:Pfizer: Honoraria; Celgene/BMS: Honoraria. Patkowska:Novartis: Honoraria. Watek:Novartis: Honoraria. Desai:Janssen Research: Research Funding; Takeda, Bristol Myers Squibb, Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees. Ritchie:Celgene: Consultancy; Novartis: Consultancy; Jazz: Consultancy; Pfizer: Consultancy; Incyte: Consultancy; Takeda: Consultancy. Guzman:Samus Therapeutics: Other: Inventor on licensed IP; Seq RX: Current holder of stock options in a privately-held company; BridgeMedicines: Research Funding. Roboz:Celgene: Consultancy, Other: travel and accommodation expenses, Research Funding; Roche: Consultancy; Mesoblast: Consultancy; Helsinn Therapeutics: Consultancy; AbbVie: Consultancy, Other: travel and accommodations, Research Funding; Bristol Myers Squibb: Consultancy; Astellas: Consultancy; Amgen: Consultancy; Astex Pharmaceuticals: Consultancy, Other: Travel and Accommodation expenses, Research Funding; CTI: Research Funding; Pfizer: Consultancy, Honoraria, Other: Travel and accommodation expenses; Bristol Myers Squibb: Consultancy; Genentech/Roche: Consultancy, Other: Travel and accommodation expenses; Celltrion: Consultancy, Other: Travel and accommodation expenses; Sandoz: Consultancy, Other: Travel and accommodation expenses; Karyopharm Therapeutics: Research Funding; Amgen: Consultancy, Other: travel; Clovis Oncology: Other: Travel and accommodation expenses; Takeda: Consultancy; Janssen: Consultancy, Other: travel and accommodation expenses, Research Funding; Otsuka: Consultancy; Agios: Consultancy, Research Funding; Novartis: Consultancy, Other: Travel and accommodation expenses, Research Funding; Actinium: Consultancy; Amphivena Therapeutics: Other: Travel and accommodation expenses, Research Funding; Eisai: Other: Travel and accommodation expenses; MedImmune: Consultancy, Research Funding; Mofitt Cancer Center: Research Funding; MEI Pharma: Consultancy, Research Funding; Daiichi Sankyo: Consultancy; Sunesis Pharmaceuticals: Other: Travel and accommodation expenses, Research Funding; GlaxoSmithKline: Consultancy; Agios: Other: travel, Research Funding; Array BioPharma: Other: Travel and accommodation expenses; Bayer: Consultancy, Other: Travel and accommodation expenses; Jazz: Consultancy, Other: travel; Jasper Therapeutics: Consultancy; Onconova Therapeutics: Research Funding; Tensha Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.