Abstract
Background: Mosunetuzumab (Mosun) is a CD20xCD3 bispecific antibody that engages T cells and B cells to induce a synthetic synapse leading to T-cell activation and production of cytokines involved in immune-directed B-cell killing. Mosun is being developed as a fixed-duration, off-the-shelf therapy for the treatment of non-Hodgkin lymphoma (NHL). A Phase I/II study (NCT02500407) is investigating Mosun monotherapy in patients (pts) with relapsed or refractory NHL (aggressive NHL [aNHL; n=278]: DLBCL, MCL, RS, transformed FL, FL3b; indolent NHL [iNHL; n=168]: FL, MZL, SLL) using fixed (Group A; n=33) and step-up (Group B; n=414) dosing schedules. We report exploratory pharmacodynamic (PD) biomarker analyses from this study across dosing cohorts and histologies in relation to mechanism of action, dose, safety, and clinical activity.
Methods: In Group A, the target dose (TD) of intravenous Mosun was administered on Cycle (C) 1 Day (D) 1 (0.05-2.8mg). In Group B, step-up doses (SUD) were administered on C1D1 (0.4-1mg), C1D8 (1-2mg) and the TD on C1D15 (2.8mg-60mg). In both groups, TD was given on D1 of subsequent cycles (up to C8 for pts achieving a complete response or C17 for pts achieving partial response or stable disease). The recommended dose was confirmed as C1D1 (1mg), C1D8 (2mg), C1D15 and C2D1 (60mg) and C3D1-C8/C17 (30mg). Cytokines (IL-6, INF-γ, TNF-α, IL-2, IL-10) were measured by ELISA or SIMOA assays and peripheral blood (PB) immune-cell subsets by flow cytometry. Baseline (BL) tumor biopsies and on-treatment (on-Tx) biopsies collected between C1D15 and C3D1 were assessed by CD8 immunofluorescence (IF) and RNAseq.
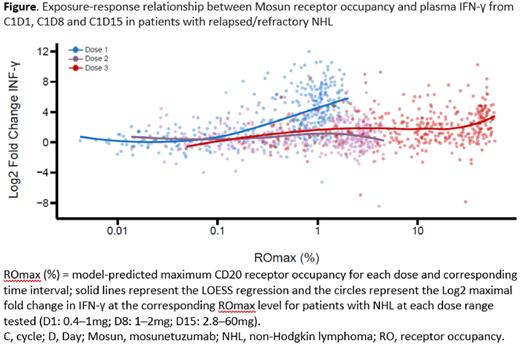
Results: In Group A, cytokine release was observed at Mosun doses of ≥0.4mg. Cytokine release was acute and transient, occurring early after Mosun administration and returning to BL levels by 24 hours. Despite a low to moderate correlation across cytokines at BL, a similar pattern of cytokine secretion was observed following administration of Mosun. High levels of cytokines were detected in pts who experienced cytokine release syndrome (CRS). In Group B, SUD was initiated as a CRS mitigating strategy, using the identified, pharmacodynamically active dose as the initial dose. Although a second cytokine peak was observed at C1D15 during SUD, with a trend towards higher peaks with increasing Mosun doses, significantly higher doses could be administered without corresponding cytokine release. While Mosun exhibited linear pharmacokinetics, dose-dependent PD relationships can be confounded by variables that affect target binding including residual anti-CD20 monoclonal antibodies from prior treatment. Taking CD20 receptor occupancy into account, exposure-response analyses using IL-6 or INF-γ confirmed that SUD ameliorates cytokine release to enable higher Mosun dosing to achieve clinically active exposure (Figure).
T-cell margination, activation (CD69), and proliferation (Ki67) were observed in CD4+ and CD8+ T cells in a time-dependent manner that paralleled PD changes observed with cytokines in both aNHL and iNHL. CD4+ and CD8+ T-cell activation was observed after the initial Mosun dose and to a lesser extent with subsequent doses. In contrast, B-cell depletion was observed early after initial Mosun administration and was sustained throughout treatment.
At BL, higher levels of PB B cells were associated with improved response in pts with aNHL. However, this may reflect prognostic features and prolonged time since prior therapy. On-Tx increases in activated T-cell subsets (% CD4+DR+GzB+ and % CD4/8 Tn DR+) were also associated with improved response. Changes in biomarker levels were observed predominantly with the first dose of Mosun; associations with response were primarily seen in pts with aNHL vs iNHL, possibly reflective of the distinct immune biology. Importantly, PD changes observed in PB were also observed in tumors. Increases in CD8+ T cells (CD8 IF) in on-Tx tumor biopsies were predominantly, but not exclusively, reported in pts who achieved a response. Consistent with PB observations, changes in RNA levels of CD8, cytokines and markers of T-cell activation (i.e. GZMB and TIM3) were also observed by RNAseq at the tumor site.
Conclusions: PD biomarkers of Mosun in PB and tumor biopsies confirm immune-cell activity associated with Mosun treatment and demonstrate the benefit of SUD as a CRS mitigation strategy to enable administration of clinically active doses.
Disclosures
Huw:Genentech, Inc.: Current Employment. Li:F. Hoffmann La Roche, Ltd.: Current equity holder in publicly-traded company; Genentech, Inc.: Current Employment. Belousov:F. Hoffmann La Roche, Ltd.: Current Employment, Current holder of stock options in a privately-held company. Ramesha:Genentech, Inc.: Current Employment. Wei:Genentech, Inc.: Current Employment; F. Hoffmann La Roche, Ltd.: Current equity holder in private company, Current holder of stock options in a privately-held company, Patents & Royalties. Yin:F. Hoffmann La Roche, Ltd.: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Genentech, Inc.: Current Employment, Patents & Royalties. Turner:Genentech, Inc.: Current Employment; F. Hoffmann La Roche, Ltd.: Current holder of stock options in a privately-held company; GSK: Ended employment in the past 24 months. Penuel:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company.
OffLabel Disclosure:
Mosunetuzumab is a CD20xCD3 bispecific antibody that redirects T cells to engage and eliminate malignant B cells. Mosunetuzumab is an investigational agent in the United States.
Author notes
Asterisk with author names denotes non-ASH members.