Abstract
Introduction: Asciminib is the 1st BCR::ABL1 inhibitor that works by Specifically Targeting the ABL Myristoyl Pocket (STAMP). Based on the phase 3 ASCEMBL trial, asciminib was FDA approved for the treatment of adult pts with Philadelphia chromosome-positive CML-CP after ≥2 TKIs and for pts with the T315I mutation.
In ASCEMBL, after ≥2 years of follow-up, asciminib continues to demonstrate superior efficacy, safety, and tolerability compared with bosutinib (BOS), with more pts on asciminib remaining on treatment and continuing to benefit from therapy over time. In a preplanned subgroup analysis, major molecular response (MMR; BCR::ABL1IS ≤0.1%) rates were consistently higher with asciminib than BOS across major demographic and prognostic subgroups. Here we describe exploratory analyses aimed to characterize the efficacy of asciminib over time and compared with BOS (data cutoff for ASCEMBL wk 96 analysis: October 6, 2021).
Methods: Eligible pts with intolerance or treatment failure (lack of efficacy) per European LeukemiaNet (ELN) 2013 recommendations were randomized 2:1 to asciminib 40 mg twice daily or BOS 500 mg once daily, stratified by baseline major cytogenetic response status. Per ELN 2013, pts with BCR::ABL1IS >10% at wk 24 were considered to have lack of efficacy and discontinued therapy. Analyses included molecular response at different time points according to baseline BCR::ABL1IS, cumulative incidence of MMR/BCR::ABL1IS ≤1% in nonresponder pts by wk 24, and logistic regression to assess baseline characteristics as predictive factors of MMR achieved early (by wk 24) vs late (after wk 24). MMR rate at wk 96 according to prior TKIs and reason of discontinuation was analyzed with asciminib and BOS.
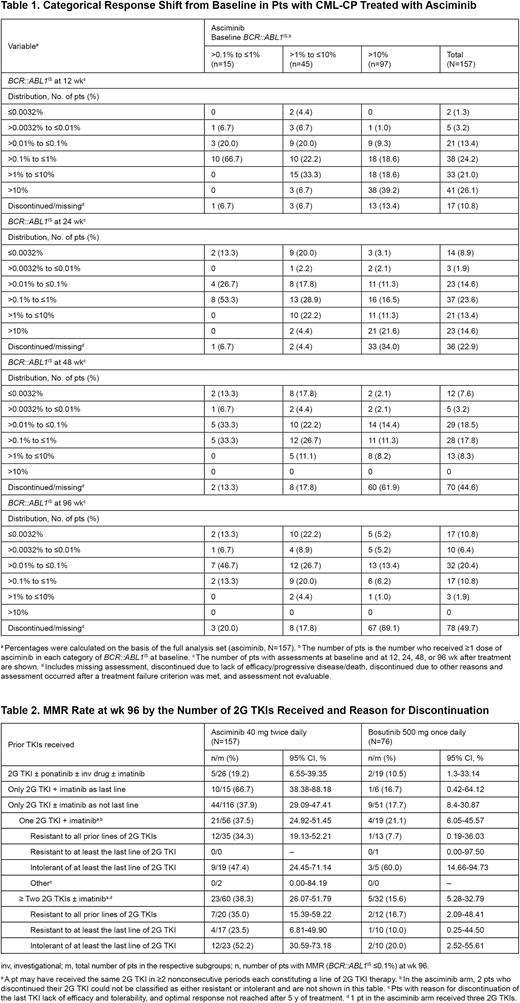
Results: Of 60 pts on asciminib with BCR::ABL1IS ≤10% at baseline, 18 (30.0%), 24 (40.0%), and 36 (60.0%) reached MMR at wk 12, 24, and 96, respectively, and 38 (63.3%), 45 (75.0%), and 47 (78.3%) reached BCR::ABL1IS ≤1% at wk 12, 24, and 96, respectively. Of 97 pts with BCR::ABL1IS >10% at baseline, 10 (10.3%), 16 (16.5%), and 23 (23.7%) reached MMR at wk 12, 24, and 96, respectively, and 28 (28.9%), 32 (33.0%), and 29 (29.9%) reached BCR::ABL1IS ≤1% at wk 12, 24, and 96 (Table 1). Per protocol, twenty-one (21.6%) pts had BCR::ABL1IS >10% at wk 24 and discontinued treatment per protocol.
Of 18 pts on asciminib with BCR::ABL1IS >1% by wk 24, the estimated cumulative incidence of BCR::ABL1IS ≤1% (95% CI) was 22.2% (6.5%-43.6%) by 1 year and 38.9% (16.4%-61.0%) by 2 years. Of 56 pts on asciminib without MMR by wk 24 and who were still on asciminib after wk 24, the estimated cumulative incidence of MMR (95% CI) was 17.9% (9.1%-29.0%) by 1 year and 37.9% (25.1%-50.6%) by 2 years. No baseline characteristics were identified as independent predictive factors of early vs late MMR.
Overall MMR rates at wk 96 were higher with asciminib than BOS regardless of the last prior TKI received (imatinib, 55.6% vs 14.3%; nilotinib, 40.9% vs 35.7%; dasatinib, 37.1% vs 10.5%; radotinib, 50.0% vs 0%; ponatinib, 20.0% vs 11.8%) and higher in pts who discontinued their last TKI due to intolerance (asciminib, n=59; BOS, n=22) vs resistance (asciminib, n=95; BOS, n=54). A higher proportion of pts who were resistant to the prior lines of 2nd-generation (2G) TKIs received before randomization, achieved MMR with asciminib vs BOS, respectively, at wk 96: 34.3% vs 7.7% with 1 prior 2G TKI; 35.0% vs 16.7% with ≥2 prior 2G TKIs (Table 2).
Conclusions: Responses continued to deepen over time with asciminib in pts with CML-CP after ≥2 prior TKIs, with additional pts achieving MMR at later time points. Pts on asciminib achieved MMR regardless of the last TKI and reason for discontinuation (resistance/intolerance), and overall MMR rates at wk 96 were higher with asciminib than BOS across all last prior TKIs. In pts with resistance to all prior lines of 2G TKIs, MMR rates at wk 96 were higher with asciminib than BOS and comparable to the previously published MMR rate of all pts randomized to receive asciminib (37.6 %). Overall, these results demonstrate that pts who continued asciminib therapy beyond wk 24 have a considerable probability of achieving MMR at later time points. The efficacy of asciminib after treatment failure on 2G TKIs supports a new standard of care for pts with CML-CP for whom ≥2 prior TKIs have failed.
This study is sponsored by Novartis.
Disclosures
Hughes:Novartis: Consultancy, Research Funding; Enliven: Consultancy, Research Funding; BMS: Consultancy, Research Funding. Rea:Pfizer: Consultancy; Incyte: Consultancy; Novartis: Consultancy. Boquimpani:Novartis, Jansen, Pint Pharma, EMS: Honoraria. Minami:Bristol-Myers Squibb Company: Honoraria; Novartis Pharma KK: Honoraria; Pfizer Japan Inc.: Honoraria; Takeda: Honoraria. Mauro:Sun Pharma/SPARC: Research Funding; AbbVie, Bristol Myers Squibb, Novartis, Pfizer, Takeda: Consultancy, Honoraria, Other: Travel, accommodation, expenses , Research Funding. Cortes:Bristol Myers Squibb: Consultancy, Research Funding; Sun Pharma: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current equity holder in private company; Gilead: Consultancy; Forma Therapuetic: Consultancy; Abbvie: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Kartos: Research Funding. Apperley:Pfizer: Honoraria, Research Funding, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. García Gutiérrez:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Research Funding. Kapoor:Novartis: Current Employment, Current equity holder in private company. Allepuz:Novartis: Current Employment. Quenet:Novartis: Current Employment. Zhang:Novartis: Current Employment. Hochhaus:Pfizer: Research Funding; Incyte: Research Funding; BMS: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.