Abstract
Multiple myeloma (MM) is a cancer characterized by the expansion of clonal plasma cell (PCs) in the bone marrow (BM). While outcomes for patients with MM have improved over the last several decades, it is still considered an incurable disease and mechanisms of relapse are still being elucidated. As the BM is a well-defined physiological niche for PCs that is often dysregulated in MM, we investigated how BM immune microenvironment signatures present at remission may influence clinical outcomes otherwise insufficiently explained by clinical characteristics or measurable residual disease (MRD) status.
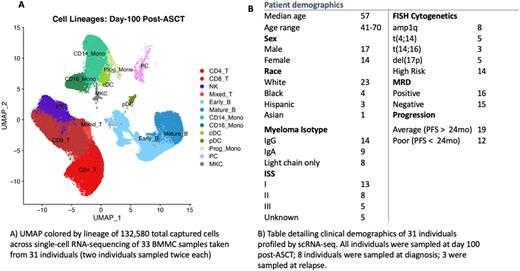
We conducted 3’ single-cell RNA sequencing (scRNAseq) of aspirated bone marrow mononuclear cells (BMMCs) sampled from 31 MM patients at day 100 following autologous stem cell transplant (ASCT) with paired MRD testing by next generation sequencing. In addition, samples from 8 of the patients at diagnosis and 3 at relapse were assessed, as were samples from 4 healthy donors.16 of 31 patients were MRD+ at day 100 post-transplant; among these, 6 had poor outcome (PO; progression-free survival <2.5yrs post-ASCT), and 10 average outcomes (AO; PFS >2.5yrs). The remaining 15 patients were MRD- post-transplant, 6 of whom having had PO and 9 AO.
scRNAseq of 45 samples have yielded a total of 228,599 cells, with an average capture of 4,450 cells per sample and 1,266 median genes per cell. Major lymphoid and myeloid lineages are represented, with the largest populations being T cells, CD14+ monocytes, B cells, and NK cells. In comparison to NDMM/relapse samples and healthy donor BMMCs from 4 individuals, post-ASCT samples exhibit pronounced enrichment for earlier B cell lineages with gene expression indicative of active cell cycling, as well as elevated CD8+:CD4+ T cell ratios with differential enrichment of specific effector/memory subsets. Naïve T cells and memory B cells are especially depleted post-ASCT. These differences likely reflect previously reported lineage skewing due to post-ASCT immune repopulation. Across our cohort, we see T, B, and CD16+ monocyte subclusters composed primarily (>70% ) of cells from either PO or AO individuals, suggesting the existence of outcome-associated immune subpopulations. Regardless of MRD status, elevated NK count (in both CD56 bright and dim) is consistently seen in post-ASCT samples from PO individuals, suggesting a possible association of NK activity with earlier relapse. Paradoxically, depletion of a CD8+ effector subset distinguished by high CCL5, NKG7, GZMH, GNLY, and IL32 is also consistently seen in PO samples. T, NK, and B cells from AO samples consistently exhibit FOS/JUN upregulation relative to those from PO samples, indicative of differential AP-1 transcription factor activity between the groups, though JUN is upregulated in all patient samples relative to healthy donors. Preliminary analysis of senescence markers in this cohort suggests that there may be several age-related confounders when comparing patient BM against younger healthy donor BM; JUN is one example of this. Increased PCs were detected in several MRD-negative samples compared to healthy donor BM, which suggests possible persistence of disease phenotypes not captured by MRD testing. By reconstructing VDJ clones from scRNAseq reads, we are able to show differences in immunoglobulin clonal diversity of patient PCs, B, and T cells that may further elucidate the true disease burden in post-ASCT samples. Overall, this study enhances our understanding of how immune surveillance and interaction may influence remission duration in MM.
Disclosures
Jayasinghe:Multiple Myeloma Research Foundation: Consultancy; Wugen: Consultancy. Vij:oncopetideslegend: Honoraria; adaptive: Honoraria; BMS: Honoraria, Other: Grant support; Sanofi: Honoraria, Other: Grant support; GSK: Honoraria; Beigene: Honoraria; Takeda: Honoraria, Other: Grant support; Janssen: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.